 54 142 Pill Images (White / Round)
54 142 Pill Images (White / Round)Copyright © 2018 by RxList Inc. RxList does not provide medical advice, diagnosis or treatment. Methadose methadose (methadone hydrochloride tablets) Find the lowest prices on MethadoseTM (methadone hydrochloride) Oral tablets, USPMethadoseTM WARNINGWARNINGABUSE POTENTIAL, LIFE-THREATENING RESPIRATORY DEPRESSION, VIFE-THREATENING QT PROLONGATION, ACCIDENTAL EXPOSURE, and TREATMENT FOR OPIOID ADDICTIONABUSE POTENTIAL, LIFE-THREATENING RESPIRATORY DEPRESSION, VIFE-THREATENING QT PROLONGATION, ACCIDENTAL EXPOSURE, and TREATMENT FOR OPIOID ADDICTIONAbuse Potential Methodontics contain methadone, opioid agonist and List II controlled substance with liability for abuse similar to other opioid agonists, legal or illicit [see Warnings and ]. Evaluate each risk of abuse or addiction of the patient before prescription Methadose. Risk of opioid abuse increases in patients with a family or staff history of substance abuse (including drug or alcohol abuse or addiction) or Mental illness (e.g., major depressive disorder). Routine monitoring of all Patients Measured for signs of abuse, abuse and addiction during treatment [see ].Methadose contains methadone, an opioid agonist and List II controlled substance with liability for abuse similar to other opioid agonists, legal or illicit [see Warnings and ]. Evaluate each risk of abuse or addiction of the patient before prescription Methadose. Risk of opioid abuse increases in patients with a family or staff history of substance abuse (including drug or alcohol abuse or addiction) or Mental illness (e.g., major depressive disorder). Routine monitoring of all Patients Measured for signs of abuse, abuse and addiction during treatment [see ]. Respiratory depression that threatens life Respiratory depression, including fatal cases, have been reported during the initiation and conversion of patients to Methadose, and even when the medication has been used as recommended and has not been misused or abused [see Warnings And... The proper dosage and titration are essential and Methadose should only be prescribed by health professionals who are knowledgeable in the use of powerful opioids for the management of chronic pain. Monitor for respiratory depression, especially during the initiation of Methadose or later increased dose. Methadose peak respiratory depressive effect occurs later, and persist longer than the analgesic peak effect, especially during the initial period of dosage. Respiratory depression, including fatal cases, have been reported during the initiation and conversion of patients to Methadose, and even when the medication has been used as recommended and has not been misused or abused [see Warnings And... The proper dosage and titration are essential and Methadose should only be prescribed by health professionals who are knowledgeable in the use of powerful opioids for the management of chronic pain. Monitor for respiratory depression, especially during the initiation of Methadose or later increased dose. Methadose peak respiratory depressive effect occurs later, and persist longer than the analgesic peak effect, especially during the initial period of dosage. QT prolongation that threatens life PT prolongation of the interval and severe arrhythmia (storages of points) have occurred during methadone treatment. Most cases involve patients be treated for pain with large daily doses of methadone, though cases have been reported in patients who receive commonly used doses Opioid addiction maintenance treatment. Near patient monitoring for changes in the heart rate during the initiation and titration of Methadose. PT prolongation of the interval and severe arrhythmia (torredes of points) have occurred during methadone treatment. Most cases involve patients be treated for pain with large daily doses of methadone, though cases have been reported in patients who receive commonly used doses Opioid addiction maintenance treatment. Near patient monitoring for changes in the heart rate during the initiation and titration of Methadose. Accidental exposureIngestion of Methadose, especially in children, may result in a fatal overdose of methadone [see ATTENTION AND ]. Accidental ingestion of Methadose, especially in children, may result in a fatal overdose of methadone [see ATTENTION AND ].Conditions for the distribution and use of methadone products For the treatment of opioid addiction For detoxification and maintenance of opioid dependence, Methadone should be administered in accordance with treatment standards referred to in section 8 of the CFR, including limitations on unsupervised administration [see ] For detoxification and maintenance of opioid dependence, Methadone should be administered in accordance with treatment standards referred to in section 8 of the CFR, including limitations on unsupervised administration [see ] DESCRIPTIONMethadone hydrochloride is chemically described as 6-(dimethylamino)-4,4-diphenyl-3-hepatanona chloride. Methadone hydrochloride is a white and crystalline material that is water-soluble. Methadone hydrochloride has a melting point of 235°C, and a pKa of 8.25 in water at 20°C. It's... octanol / water partition coefficient to pH 7.4 is 117. One solution (1:100) in the water has a pH between 4.5 and 6.5. It has the following structural formula: MethadoseTM Oral Tablets (Methadone Chlorhydrate tablets) USP) Each contains 5 or 10 mg methadone chloride, USP and the following inactive ingredients: dibic calcium phosphate, microcrystalline cellulose, magnesium stearate, colloidal silicon dioxide, pregelatinized starch and Acid. INDICATIONSMethadoseTM Oral Tablets (metadona hydrochloride) Paintings, USP) is indicated for:Limitations of UseMethadose is not for use: Conditions of distribution and use of methadone products For the treatment of opioid addictionMethadone products when used for the treatment of opioids addiction in detoxification or maintenance programmes will only be dispensed by opioid treatment programs (and agencies, practitioners or institutions by formal agreement with the program sponsor) certified for substance abuse and Administration of Mental Health Services and approved by the State designated authority. Certified treatment programs will be dispensed and used in methadone only in oral form and according to the treatment requirements stipulated in the Federal Opioid treatment standards (42 CFR 8.12). See below for important regulation exceptions to the general certification requirement to provide agonist opioid treatment. Failure to comply with the requirements of these standards can lead to criminal prosecution, drug supply, revocation of approval of the program, and operation of exclusion of orders from the program. Regulatory Exceptions to the General Requisition Certification to provide agonist opioid treatment: During patient care, when the patient was admitted for any condition other than concurrent opioids Addiction (pursuant to 21CFR 1306.07(c)), to facilitate the treatment of primary diagnosis admitting). During an emergency period of no more than 3 days while the ultimate attention of addiction is sought in an appropriate license ease (computer to 21CFR 1306.07(b)). SLIDESHOWDOSAGE AND ADMINISTRATION Initial symptoms for pain managementConsider the following factors when selecting an initial Methadose dose: Also consider the following important factors differentiate methadone from other opioid analgesics: Method is given at a frequency of 8 to 12 hours. Initiate small-dose method therapy, no more than just 2.5 mg to 10 mg every 8 to 12 hours. To keep fit, more frequent administration may be necessary. Supervising patients close to detect signs respiratory and depression. Use a 1:2 mg conversion ratio for parenteral to oral methadone (for example, 5 mg of methadone parenteral at 10 mg of oral methadone). Conversion rates published for other opioids to methadone you can overestimate the dose of methadone. Deaths have occurred in opioid tolerant patients during conversion to methadone. Deaths have occurred in opioid tolerant patients during conversion to methadone. conversion ratios in many commonly used equianalgeic doses Tables are based on unique dose comparisons in patients not tolerant of the opioid effects and are not applied in the establishment of the conversion of opioids Methadone tolerant patients for chronic use. In the case of a single dose administration, initiation, duration and power of analgesic action Methadone is comparable to morphine. Incomplete cross tolerance can is more toxic than expected. In addition, with repeated doses, Methadone power increases due to systemic accumulation. The conversion ratio between methadone and other opioids varies drastically depending on the use of reference opioids (morphine value) as is shown in the table below. The dose conversion scheme below (Table 1) is derived from several consensus guidelines to convert patients to methadone morphine. Conversion guidelines issued to determine equivalent morphine doses for patients who convert from other opioids. Table 1: Oral morphine to oral methadone conversion Chronic Administration Table 1: Oral morphine to oral methadone conversion Chronic Administration Total Daily Baseline Dose Daily Methadone Requirement as a percentage of the total daily morphine dose (100 mg) 20% to 30% 100 to 300 mg 10% to 20% 300 to 600 mg 8% to 12% 600 mg to 1000 mg 5% to 10% √1000 mg (c) 5 per cent Divide the total daily dose of methadone derived from the table above to reflect the planned dosing schedule (i.e., for administration every 8 hours, divide the total daily dose of methadone by 3). The dose of methadone equianalgesic varies not only between patients, but also within the same patient, depending on the base morphine (or another opioid dose. Table 1 has been included to illustrate this concept and provide a recommendation for a starting point for the conversion of opioids. In addition to those recommendations, patient consideration:Treatment and Maintenance of Pain TherapyIndividually titrate Methadose at a dose that provides appropriate analgesia and minimizes adverse reactions. Continuous revaluation of patients received Measures to evaluate the maintenance of pain control and relative incidence of adverse reactions. During chronic therapy, especially for pain not related to cancer (or pain associated with other terminal diseases), periodically reevaluates the constant need to use opioid analgesics. If the level of pain increases, try to identify the increased pain source, while adjusting Methadose dose to decrease level of pain. Because the plasma concentrations of fixed status are approximate within 24 to 36 hours, Metdose settings can be made every 1 to 2 days. Patients who experience advance pain may require dose adjustment or rescue medication with a small dose of an immediate release medication. If there are signs of excessive adverse reactions related to opioids observed, the following dose can be reduced. Adjust the dose to get a suitable one balance between pain management and opioid-related adverse reactions. The endpoint of titration is achievement of adequate pain relief, balanced against tolerability of adverse opioid reactions. If a patient develops adversely related to intolerable opioids reactions, methadone dose, or dosing interval, may need to be adjusted. Discontinued method for painWhen a patient no longer requires therapy with the method pain, use a gradual downward titration, from dose every two to four days, prevent signs and symptoms of withdrawal in the physically dependent patient. Do not abruptly decolgate Methadose. Induction/initial dose for detoxification and maintenance Opioid Addiction Treatment For detoxification and maintenance of opioid dependence Methadone should be administered in accordance with treatment standards referred to in section 8.12 of the CFR, including limitations to the administration. Manage the initial dose of methadone under supervision, when there are no signs of sedation or poisoning, and the patient shows withdrawal symptoms. An initial dose of 20 to 30 mg of Methadose often be enough to suppress. The initial dose should not exceed 30 mg. To make dosing settings of the same day, make the patient wait 2 to 4 hours for subsequent evaluation, when the maximum levels have been reached. Proportion an additional 5 to 10 mg of Methadose if withdrawal symptoms have not been suppressed or if the symptoms reappear. The total daily dose of Methadose in the first day of treatment should not normally exceed 40 mg. Adjust the dose on the first week of treatment based on the control of withdrawal symptoms at the time of the expected peak activity (e.g. 2-4 hours after dosing). When adjusting dose, take into account that methadone levels will accumulate in the first dosage days; deaths occurred in early treatment due to cumulative Effects. Instruct patients that the dose "conserves" for a longer period time as the methadone tissue stores accumulate. Use lower initial doses for patients whose tolerance is It is expected to be low at the treatment entrance. Any patient who has not taken opioids for more than 5 days may not be tolerant. Do not determine the initial dose based on previous treatment episodes or dollars spent per day on illicit drugs use. For a short stabilization course followed by a period medically supervised retirement, order the patient at a total daily dose of approximately 40 mg in divided doses to achieve an adequate level of stabilization. After 2-3 stabilization days, gradually decreasing the dose of Methadose. Decrease Methadose doses daily or at intervals of 2 days, keeping the amount of Measure enough to maintain withdrawal symptoms at a tolerable level. Hospitalized patients can tolerate a daily reduction of 20% of the total daily dose. Outpatients may need a slower schedule. Treatment of Titration and Maintenance of Detoxification of Opioid Dependency Titrate patients in maintenance treatment at a dose that prevents symptoms of opioid withdrawal for 24 hours, reduces hunger or the craving of drugs, and blocks or mitigates the euphoric effects of self-administered opioids, ensure that the patient is tolerant for the sedative effects of the methadone. More commonly, clinical stability is achieved in doses between 80 and 120 mg/day. Medically supervised retirement after a period Maintenance treatment for opioid addiction There is considerable variability in the appropriate rate Methadone cincuentene in patients who choose medically supervised methadone withdrawal treatment. Dosage reductions should be less than 10% of those established dose of tolerance or maintenance, and intervals of 10 to 14 days should pass between dose reduction. Approve patients at high risk of relapse into illicit drugs use associated with the interruption of the methadone maintenance treatment. Risk of relapse in patients in maintenance of methadone Opioid Addiction TreatmentOpioid suppression may lead to development of opioids symptoms of opioid withdrawal [see ]. Symptoms of opioid withdrawal have been associated with a Increased risk of relapse into illicit drug use in susceptible patients. Considerations for acute pain management during methadone Maintenance TreatmentPatients in Methadone Maintenance Treatment for Opioids dependence that experiences physical, postoperative or other acute pain pain cannot be expected to derive pain from your current dose of pain Methadone. These patients should be given painkillers, including opioids, in doses that would otherwise be indicated for patients not treated with methadone with similar painful conditions. When opioids are required for management acute pain in methadone maintenance patients, something higher and/or more frequent doses of what would be the case of non-tolerant will often be required patients due to opioid tolerance induced by methadone. Dosage adjustment during pregnancy Methadone authorization can be increased during pregnancy. During pregnancy, a woman's methadone dose may need to be increased or increased the dosage interval decreased. Methadone should be used in pregnancy only if potential benefit justifies the potential risk for the fetus [see ]. How SUPPLIEDDosage Forms and Strengths Methadose Oral Tablets (metadona hydrochloride tablets USP) are available at 5 mg and 10 mg strength dose. 5 mg tablets are white, tablets scored (identified methods 5). 10 mg tablets are white, scored tablets (identified methods 10). Methadose storage and handling contains methadone which is a control substance. As fentanyl, morphine, hydromorphone, andoxymorphone, methadone is controlled under List II Federal Controlled Substances Act. The method can be directed for theft and fun for criminals [see Warnings and ]. AND Stay closed. Dispensing in a tight, light resistant container. Store at 20° to 25°C (68° to 77°F) [see USP Control Room temperature]. USP Controlled Room Temperature How MethadoseTM Oral Tables (methadone chlorhydrate tablets) are supplied USP:MethadoseTM Oral Tablets (metadona hydrochloride tablets USP:5 mg of white color, annotated tablets (identified methods 5)5 mg bottles of 100................NDC 0406-6974-34NDC10 mg of white color, annotated tablets (identified methods 10)10 mg bottles of 100.......................................................................................... DEA order form required. Reviewed: 07/2012. Distributed by: Mallinckrodt, IncSIDE EFFECTS The following serious adverse reactions and/or conditions are discussed in other locations of labelling: WARNING AND WARNING AND WARNING AND AND ADMINISTRATION AND WARNING AND ADMINISTRATION AND ADMINISTRATION The main dangers of methadone are respiratory depression and, to a lesser degree, systemic hypotension. Respiratory strike, shock, heart arrest, and death has occurred. The main dangers of methadone are respiratory depression and, to a lesser degree, systemic hypotension. Respiratory strike, shock, heart arrest, and death has occurred. The most frequent adverse reactions include dizziness, dizziness, sedation, nausea, vomiting and sweating. These effects appears to be more prominent in outpatients and those who are not suffering severe pain. In such individuals, the lowest doses are recommended. Other adverse reactions are the following: Body as a whole: (weakness), edema, headache Body as a whole:Cardiovascular: arrhythmias, large rhythms, , , ECG abnormalities, extrasystoles, flushing, , , , prolongation of the QT interval, , wave T investment, , point torsades, , Cardiovascular: Central Nervous System: agitation, confusion, disorientation, , insomnia, hallucinations, seizures, visual disturbances Central nervous system:Endocrine: Endocrine:Gastrointestinal: abdominal pain, biliary tract spasm, constipation, , Gastrointestinal: Hematological: reversible has been described in opioid addicts with chronic hematologic:Metabolic: , weight gainMetabolic: Renal: antidyuretic effect, urinary retention or hesitancyRenal:Reproductive: , reduced and/or power, reduced ejaculate volume, seminal vesicle and reduced prostate secretions, decrease , abnormalities in the Reproductive sperm:Respiratory: , respiratory depressionRespiratory: Ski and subcutaneous tissue: , , other skin rashes, and rarely, hemorrhagic hivesSkin and Tissue subcutaneous:Hypersensitivity: has been reported with ingredients contained in Methadose. Advise patients how to recognize such a reaction and when to look for medical care. Hypersensitivity: Staying in a stabilized dose: For long administration of , as in a methadone maintenance treatment program, constipation and sweat often persist and hypogonadism, serum decrease and reproductive effects are considered to be related opioid use. Maintenance in a stabilized dose:During the induction phase of maintenance of the methadone treatment, patients are being removed from illicit opioids and may have opioids . Monitoring patients for signs and symptoms including: , , sneezing , excessive breath, geese-flesh, fever, cooling alternating with washing, restless, irritability, weakness, anxiety, depression, dilated pupils, tremors, tachycardia, abdominal cramps, body aches, involuntary twitching and kicking movements, anorexia, nausea, vomiting, diarrhea, intestinal spasms and weight loss and consider the dose adjustment as indicated. INTERACTIONS OF QUESTIONDRUGCytochrome Interactions P450 Methadone suffers liver N-demethylation by cytochrome P450 (CYP) isoforms, mainly CYP3A4, CYP2B6, CYP2C19, and a minor extension by CYP2C9 and CYP2D6 [see ]. CLINESE PHARMACOLOGY Simultaneous use of the method and medications that induce cytochrome P450 enzymes (such as rifampicin, phenitoin, phenobarbital, carbamazepin and St. John's Wort) can lead to a reduction in Methadose's effectiveness and could precipitate a withdrawal syndrome. Carefully monitors patients receiving Methadose and a close enzyme inducer for withdrawal signs and adjust the Methadose doses accordingly. Co-management of medicines that inhibit CYP3A4 (such as ketoconazol, itraconazole, voriconazole, clarithromycin, , telithromycin) and/or drugs that inhibit CYP2C9 (such as sertraline and fluvoxamine) can cause decrease in the cleaning of the methadone, which could increase or prolong the use of adverse drugs effects and can cause fatal respiratory depression [see ]. Supervising close patients to detect respiratory or central signs nervous system depression when Methadose is prescribed with a CYP3A4 inhibitor and reduce the dose if necessary. Simultaneous use of certain protease inhibitors with CYP3A4 inhibitive activity, alone and in combination, such as abacavir, amprenavir, darunavir+ritonavir, efavirenz, nelfinavir, nevirapine, ritonavir, webprevir, lopinavir+ritonavir, saquinavir+ritonavir, and tipranvir+ritonavir, has resulted in an increase in the cleaning or decrease in the plasma levels of the methadone. This can lead to a reduction in Methadose's effectiveness and could precipitate a withdrawal Syndrome. Supervising methadone patients receiving any of these close therapies for evidence of withdrawal and adjustment effects the methadone dose accordingly. Didanosina and Stavudine: Experimental test showed that methadone decreased the area under the concentration time curve (AUC) and maximum levels for didanosine and stavudine, with a greater decrease for didanosine. The disposition of the methadone was not substantially altered. Didanosine and Stavudine: Zidovudine: Experimental tests showed that Methadone increased the AUC of , which could result in toxic effects. Zidovudine:CNS Depressants Methadose's Simultaneous Use Depressants (e.g. sedatives, hypnotics, general anesthesia, phenothiazins, other tranquilizers, alcohol and abuse drugs) may increase the risk of respiratory depression, hypotension and deep sedation or coma. Supervising patients receiving CNS and Methadose depressants for signs respiratory depression and hypotension. When such combined therapy is contemplated, reduce the initial dose of one or both agents. Deaths have been reported when the methadone has been abused in conjunction with . Potential arrhythmogens monitor patients closely for cardiac driving changes when any drug that has the potential to prolong the QT interval is prescribed along with the methadone. Pharmacodynamic interactions can occur with concomitant use of methadone and potentially arrhythmogenic agents as class I and III antiarrhythmics, some neuroleptics and tricyclic antidepressants, and calcium channel blockers. Similarly, monitor patients closely when describing methadone concomitantly with drugs capable of inducing electrolytic disturbances (hypomagnesemia, hypokalemia) that can prolong the QT interval, including diuretics, laxatives and, rarely, mineralocorticoid hormones. Opioid antagonists, mixed agonists and partial antagonists Agonists Like other muagonists, patients held in methadone may experience withdrawal symptoms when opioid antagonists, mixed /antagonists, and partial agonists. Examples of such agents are , , pentazocin, nalbuphine, butorphanol, and . Antidepressants Monoamine Oxidase (MAO) Inhibitors: Therapeutic dose of meperidine have precipitated severe reactions in concurrent patients receiving oxidase monoamine inhibitors or those who have received such agents within 14 days. Similar reactions so far have not been reported with methadone. However, if the use of methadone is necessary in such patients, a sensitivity test should be performed in which small and incremental doses are repeated Methadone is given for several hours while the patient condition and vital signs are carefully observed. Monoamine Oxidase (MAO) Inhibitors:Desipramine: Desipramine blood levels have increased with the concurrent administration of methadone. Desipramine:AnticolinergicosAnticholinergicos or other anticholinergic drugs activity when used simultaneously with opioids can result in increased risk of urinary severe retention and/or constipation, which can lead to . Monitoring patients for signs of urinary retention or reduced gastric motility when Methadose is used simultaneously with anti-ticolinergic drugs. Laboratory Test interactions The urine screens for the methadone have been reported for several drugs including diphenhydramine, doxylamine, clomipramine, chlorpromazine, tenderen, quetiapine and verapamil. Drug abuse and dependenceMethadone is a mu-agonist opioid with a responsibility for abuse similar to other opioid agonists and is a controlled substance of List II. Methadone and other opioids used have the potential to be abused and are subject to criminal diversion [see Warnings and ]. ADMINISTRATIONS AND All patients treated with opioids requires careful control for signs of abuse and addiction, from the use of opioid products carry the risk of addiction even under due medical use. Drug abuse is the intentional non-therapeutic use of sale free or prescription drugs, even once, for your psychological reward or physiological effects. Drug abuse includes but is not limited to drug abuse following examples: the use of a prescription or over-the-counter medicine to get "high", or the use of steroids to improve muscle performance and buildup. Drug addiction is a behavioral group, and physiological phenomena that develop after the use of repeated substances and include: a strong desire to take the drug, difficulties to control its use, which persists in its use despite the harmful consequences, a higher priority given to drug use other activities and obligations, increased tolerance and Sometimes a physical retreat. The behavior of "rebusing drugs" is very common in addicts and drugs abusers. Drug search tactics include emergency calls or visits near the end of the operation hours of office, refusal to undergo proper examination, testing or referral, repeated statements of lost recipes, manipulating recipes and reluctance to provide previous medical records or contact information for other medical treatment(s). "Doctor shopping" (visiting multiple prescriptors) obtaining additional recipes is common among drug users and people suffering from untreated addiction. Concern with the achievement of adequate pain Relief can be a proper behavior in a patient with poor pain control. Abuse and addiction are separate and distinct from physical dependence and tolerance. Doctors must be aware that addiction cannot be accompanied by concurrents tolerance and symptoms of physical dependence on all addicts. Plus, Opioid abuse can occur in the absence of true addiction. Method, like other opioids, can be diverted to non-medical use in illicit distribution channels. Maintenance of careful records of the limitation of information, including the amount, frequency and requests for renewal as required by state law, it is strongly recommended. Methadose's abuse poses a risk of overdose and death. This increases risk with the simultaneous abuse of methadone with alcohol and others substances. Methadone is only for oral use and should not be injected. Parenteral drug abuse is commonly associated with the transmission of infectious diseases as hepatitis and . Appropriate patient assessment and selection, appropriate prescribe practices, periodic re-evaluation of appropriate therapy and dispensation and storage are appropriate measures that help limit the abuse of opioid drugs. Babies born to mothers physically depend on opioids can also be physically dependent and may present breathing difficulties and withdrawal symptoms [see ]. Babies born to mothers physically depend on opioids can also be physically dependent and may present breathing difficulties and withdrawal symptoms [see ]. Both tolerance and physical dependence may develop during Chronic opioid therapy. Tolerance is the need to increase opioid doses to maintain an effect defined as analgesia (in the absence of progression of diseases or other external factors). Tolerance can occur both to the desired and undesirable effects of medicines, and can be developed at different rates for different Effects. Physical dependency leads to withdrawal symptoms after discontinued abruptly or significantly reduced doses of a drug. Retirement can also be precipitated through the administration of drugs with opioid antagonist activity, for example, naloxone, or agonist analgesic/ mixed antagonist (pentazocin, butorfanol, buprenorphine, nalbuphine). Physical dependence can does not occur in a clinically significant degree until after several days to weeks continued use of opioids. The methase should not abruptly suspend [see ]. If Methadose is abruptly discontinued in a physical A dependent patient, withdrawal syndrome may occur. Some or all below can characterize this syndrome: restless, tearing, rhinoceros, yawn, breath, chills, and other signs and symptoms may also develop, including irritability, anxiety, back pain, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, or heart rate. Babies born of physically opioid-dependent mothers also depend physically and may expose breathing difficulties and withdraw symptoms [see ]. Use in specific populationsWARNINGSIncluded as part of the PRECAUTIONS section. PRECAUTIONSPRECAUTIONS The method contains , an opioid agonist and a Table II controlled substance. Methadone can be abused in a similar way other opioid agonists, legal or illegal. Opioid agonists are wanted by drugs abusers and people with addiction disorders and are subject to crimes distraction. Consider these risks by describing or dispensing Methadose in situations where there is concern about increased risks of abuse, abuse or diversion. However, concerns about abuse, addiction and diversion should not prevent proper pain management. For each patient prescribed Methadose for , Assess the risk of opioid abuse or addiction before prescribing Methadose. Risk of opioid abuse increases in patients with a family or staff history of (including medicine or addiction) or mental illness (e.g., ). Patients with higher risk may still appropriately treated with modified release opioid formulations; however these patients will require intensive monitoring for signs of abuse, abuse or addiction. Routine monitoring of all patients receiving opioids for signs of abuse, abuse and addiction because these drugs pose a risk of addiction even under proper medical use. Contact the local state professional license board authority of controlled substances for information on how to prevent and detect abuses or fun of this product. Vida-Treatening Respiratory Depression Respiratory depression is the main risk of the method. Respiratory depression, if not immediately recognized and treated, can lead to respiratory detention and death. Respiratory depression of opioids manifests reduced breath and a decrease in rate associated with a "fishing" breathing pattern (deep sighs separated by abnormally long breaks). (CO2) retention of induced opioids respiratory depression can exacerbate the effects of opioid sedation. Management of respiratory depression may include close observation, support measures and use of opioid antagonists, depending on the patient's clinical condition [see ] OVERDOSAGE While grave, potentially fatal or fatal respiratory Depression can occur at any time during the use of Methadose, the risk is larger during the initiation of therapy or after a dose increase. The respiratory depressive effect methadone peak occurs later, and persists longer than the peak effect, especially during initial dosing period. Monitoring patients closely for respiratory depression when starting Methadose therapy and subsequent dose increases. Instructing patients against use by people other than the patient for whom Methadose was prescribed and to keep Methadose out of the reach of children, since inappropriate use can lead to respiratory death Depression. To reduce the risk of respiratory depression, adequate dosage and Methadose's titration is essential [see ]. Oversize Methadose dose by converting patients from other opioid the product can result in fatal overdose with the first dose. Respiratory depression has also been reported using methadone when used as recommended and not misused or abused. DINERO AND ADMINISTRATIONTo further reduce the risk of respiratory depression, consider the following: The proper dosage and titration are essential andCONTRAINDICATIONSThe QT prolongation of life Cases of prolongation of the QT interval and serious (point torches) have been observed during methadone treatment. These are cases seem to be more associated, but not limited to, more dose treatment (conference 200 mg/day). Most cases involve patients who are treated pain with large and multiple daily doses of methadone, although cases have been reported in patients who receive commonly used doses for maintenance treatment opioid addiction. In most patients in the lower doses is normally used for maintenance, medicines and/or clinical conditions such as pointed out as contributing factors. However, evidence strongly suggests that the methadone has the potential for adverse effects of cardiac conduction Some patients. Methadone effects at QT interval have been confirmed In vivo lab studies, and methadone has been shown to inhibit the heart channels in in vitro studies. Supervising patients with risk factors for development long QT interval (e.g. heart, concomitant use, hypokalemia, a history of heart conduction anomalies, and those who take drugs that affect heartbeat. QT prolongation has was also reported in patients with no previous heart history who have received high doses of methadone. Evaluate patients who develop QT prolongation while in methadone treatment for the presence of modifiable risk factors, such as concomitant medicines with heart effects, medicines that could cause abnormalities, and drugs that could act as methadone inhibitors. Just initiate methasis therapy for pain in patients to whom the expected benefit exceeds the risk of QT prolongation and development of dyrhythmias reported with high doses of methadone. The use of methadone in patients already known to have The prolonged QT interval has not been systematically studied. Accidental exposureIngestion of Methadose, especially in children, It can result in a fatal overdose of methadone. Methadone should stay out of the extent of children to prevent accidental ingestion. Older, cachetics and debilitated patients are more likely to occur in elderly people, , or debilitated patients as may have altered pharmacokinetic dues to poor fat stores, muscle, or altered cleaning compared to younger, Healthier patients. Therefore, monitor these patients closely, especially when to start and tetrating Methadose and when Methadose gets concomitant with other drugs that depress breathing. Use in Patients with Chronic Lung DiseasePatients with Significant Chronic Obstruction lung disease or , and patients with a substantial decrease respiratory reserve, , , or pre-existing respiratory depression for respiratory depression, especially when therapy and Titrating with Methadose, as in these patients, even regular therapeutic doses The methodus can decrease the respiratory unit to the point of . Consider the use of non-opioid alternative analgesics in these Patients if possible. Interactions with CNS depressants and illicit drugs, deep sedation, coma or respiratory depression can result if Methadose is used concomitantly with other CNS depressants (e.g. sedatives, axiolytic, hypnotic, neuroleptic, other opioids). When considering the use of Methadose in a patient taking a CNS depressive, evaluating the duration of the use of depressive CNS and the patient's response, including the degree of tolerance that has developed to the depression of the CNS. Plus, consider the use of the patient, if any, of alcohol or illicit drugs that cause CNS Depression. If methasis therapy will start in a patient taking a CNS depressive, starts with a dose of Methadose lower than usual and monitors patients for signs of sedation and respiratory depression and consider the use of a dose of CNS depressant concomitant [see ]. DROGAS INTERACTIONS Deaths associated with the illicit use of methadone have It often involved concomitant benzodiazepine abuse. Hypotensive EffectMethodus can cause severe hypotension including orthostatic hypotension and outpatients. There's a higher risk in patients whose ability to maintain blood pressure has already been compromised by a reduced blood volume or concurrent administration of certain CNS depressive drugs (e.g. phenothiazins or general anesthetics) [see ]. Supervising these patients for hypotension signals later starting or titrating Methadose's dose. Use in patients with head injuries or increased intracranial PressureMonitor patients taking Methadose may be susceptible to the intracranial effects of CO2 retention (e.g. those with increased testing) Intracranial pressure or brain tumors) for signs of sedation and respiratory depression, particularly when therapy begins with Methadose. Methadose can reduce the respiratory unit, and the resulting CO2 retention can increase even more Intracranial pressure. Opioids can also hide the clinical course in a patient with a . Avoid the use of the method unconsciousness or damaged coma. Use in Patients with Gastrointestinal ConditionsMethodus is contraindicated in patients with paralytic . Avoid the use of the methodus in patients with another gastrointestinal obstruction. Methadone in Methadose can cause sphincter spasm Oddi. Monitoring patients with tract disease, including , to make symptoms worse. Opioids can cause serum increases. Use in Patients with Convulsive or Convulsant DisordersMethodus can aggravate seizures in patients with seizures patients with seizure disorders, and may induce or aggravate seizures in some clinical settings. Monitoring patients with history to worsen seizure control during Methadose therapy. Avoidance of withdrawal Avoid the use of partial or mixed agonists agonist / analgesics (i.e., pentazocina, nalbuphine, and butorphanol) in patients who have received or are receiving a course therapy with a complete opioid agonist analgesic agonist, including Methadose. In these patients, partial agonists or mixed/antagonist analgesics can reduce the analgesic effect and/or may precipitate [see]. When Methadose is interrupted, the dose [see] is gradually attached. Do not abruptly decolgate Methadose. Driving and Operating Machinery Methods can harm the necessary mental or physical abilities potentially dangerous activities such as driving a car or operating machinery. It warns patients not to drive or operate dangerous machinery unless are tolerant for Methadose's effects and know how they will react to Medication. Patient guidance information See patient's labelling approved by the FDA ()See patient's labelling approved by the FDA ()tell patients that Methadose contains methadone, a The controlled substance in table II is subject to abuse. Instructing patients not share Methadose with others and take measures to protect Methadose from robbery or misuse. Life-Treatening Respiratory Depression discusses the risk of respiratory depression with patients, explaining that the risk is greater when start the Methadose or when the dose is increased. Advise patients how recognize respiratory depression and seek medical care if they are experience respiratory difficulties. Instructing patients to seek medical care immediately if experience symptoms that suggest arrhythmia (such as, near) Syncope, or syncope) when you take methadone. Instruct patients to take measures to securely store Methadose. Accidental exposure, especially in children, can cause serious damage or death. Advise patients to dispose of Methadose without using when throwing the tablets down WC. To inform patients that the concomitant use of alcohol Methods can increase the risk of respiratory depression that threatens life. Instructions patients who do not consume alcoholic beverages, as well as recipes and products containing alcohol during treatment Methadose. To inform patients that they may have severe additive effects may occur if Methadose is used with other CNS depressants, and not to use such medicines unless they are supervised by a health care provider. Instructing patients how to properly take Methadose, including the following: To inform patients that the method may cause orthostatic hypotension and syncopy. Instructing patients to recognize symptoms of low blood pressure and how to reduce the risk of serious consequences should hypotension occurs (for example, sitting or lying, getting up carefully from a sitting or lying position). To inform patients that Methadose may harm the capacity of potentially dangerous activities such as driving a car or operating heavy machinery. Advise patients not to perform such tasks until they know how He'll react to the medication. Advise patients of the potential for severe constipation, including management instructions and when to look for medical care. To inform patients who have been reported ingredients contained in Methadose. Advise patients how to recognize such a reaction and when to look for medical care. Advise female patients that Methadose can cause fetal damage and inform the prescriptor if they are pregnant or plan to become pregnant. Teach the mothers who use Methadose to see signs Methadone toxicity in your babies, including increased sleepiness (more of the usual), difficulty in breastfeeding, difficulty breathing or limping. Instructing breastfeeding mothers to speak immediately to the baby's health care provider if you notice these signs. If they cannot reach the right of the health care provider instruct them to take the baby to the emergency room or call 911 (or local emergency services). Carcinogenesis, Mutagenesis, Fertility Impermeability The results of the evaluation of carcinogenicity in B6C2F1 mice and Fischer 344 rats after dietary administration of two doses of methadone HCl They've been published. Mice consumed 15 mg/kg/day or 60 mg/kg/day for methadone Two years. These doses were approximately 0.6 and 2.5 times a daily human oral 120 mg/day dose on a basis (mg/m2). There was one significant increase in adenomas in female mice treated with 15 mg/kg/day but not with 60 mg/kg/day. Under the conditions of rehearsal, there was not a clear proof of an increase related to the treatment of incidence Neoplasms in male rats. Due to the decrease in food consumption in men at high doses, male rats consumed 16 mg/kg/day and 28 mg/kg/day of methadone by two years. These doses were approximately 1.3 and 2.3 times a human daily oral dose 120 mg/day, based on body surface comparison. Instead, female rats Consumed 46 mg/kg/day or 88 mg/kg/day for two years. These doses were approximately 3.7 and 7.1 times a human daily oral dose of 120 mg/day, based on body surface comparison. Under the conditions of the trial, there was no clear evidence for an increase related to treatment in the incidence of neoplasms in male or female rats. There are several published reports on genetic potential methadone toxicity. positive tested methadone in the in vivo mouse testing and in vivo aberration of mammals Try. In addition, the methadone tested positive in the system and Crassa Neurospora and mice ahead mutation tests. Instead, negative tested methadone in tests for chromosome breakage and disjunction sex-related lethal génical mutations in use cells feeding and injection procedures. Studies of published animals show that the treatment of methadone men can alter reproductive function. Methadone produces significant regression of sexual organs and tests of mice and male rats. Use in specific populations Pregnancy No adequate and well-controlled studies of use of methadone in pregnant women. Methadone has proven to be in hamster at doses 2 times the human daily oral dose (120 mg/day at a mg/m2 base) and mice at doses equivalent to the human daily oral dose (120 mg/day on a basis of mg/m2). Increased mortality and significant differences Behavioral tests have been reported in the male rodents descendence that were treated with methadone before pairing when compared to control animals. Methadone has been detected in human and plasma concentrations proportional to maternal and newborn urine plasma at lower concentrations that the corresponding maternal urine. Methadone should be used during pregnancy only if the potential benefit justifies the potential risk for the fetus. The provision of oral methadone has been studied in approximately 30 pregnant patients in 2nd and 3rd quarters. Total body authorization methadone increased in pregnant patients compared to the same patients or non-pregnant opioid women. The semi-life terminal Methadone is diminished during the second and third quarters. The decrease in plasma half life and greater cleaning of the methadone resulting in a smaller methadone trough levels during pregnancy can lead to withdrawal symptoms in some pregnancies Patients. The dose may need to increase or the dosage interval decreased in pregnant patients receiving methadone to achieve therapeutic effect [see ]. Babies born to mothers who have been taking opioids regularly before delivery can be physically dependent. Beginning of withdrawal symptoms in babies is usually in the first days after birth. Newborn monitor for signs of withdrawal and symptoms including: irritability and excess crying, tremors, active reflexes, increase, increased feces, sneezing, vomiting and fever. Neonatal intensity withdrawal syndrome is not always correlated with the maternal dose or duration of maternal exposure. The duration of withdrawal signs may vary from a few days to weeks or even months. There is no consensus on the appropriate management of the removal of infants [see Use in Specific Populations]. Use in specific populationsReported studies have generally compared the benefit of risk of untreated addiction to illicit drugs; relevance of these findings for methadone prescribed pain patients during pregnancy It's not clear. Pregnant women involved in methadone maintenance programs have been Notified that they have improved significantly and led to lower incidence of obstetric and fetal complications and neonatal morbidity and mortality compared to women who use illicit drugs. Several factors, including the use of illicit drugs, infections and psychosocial drugs circumstances, complicate the interpretation of child investigations of women who take methadone during pregnancy. Information is limited dose and duration of the use of methadone during pregnancy, and most of the maternal exposure It seems to happen after the first trimester of pregnancy. A review of the publication methadone experience data during pregnancy The Information System (TERIS) concluded that the use of the methadone during the period pregnancy as part of a supervised therapeutic regime is unlikely to have a risk teratogen (quality and quality of data evaluated as "limited to fair"). However, data are insufficient to claim that there is no risk (TERIS, the last considered in October 2002). A number of cases of 101 pregnant women, opioid-dependent women who were detoxified with opioids the methadone showed no greater risk in the second Trimester or premature delivery in the 3rd quarter. Recent studies suggest a increased risk of premature birth in women who depend on opioids exposed to methadone during pregnancy, although the presence of confusing factors makes It is difficult to determine a causal relationship. Several studies have suggested that babies born of drug addicted women treated with methadone throughout or part of pregnancy have decreased fetal growth with weight reduction, length and/or circumference of the head compared to controls. This growth deficit does not appear to persist later. Children prenatally exposed to methadone have been reported to show mild but persistent deficits in performance in psychometric and behavioral tests. In In addition, several studies suggest that children born to women who depend on opioids exposed to methadone during pregnancy may have a higher risk of visual development anomalies; however, a causal relationship has not been assigned. There are contradictory reports on whether child death was sustained Syndrome occurs with a higher incidence in children born of treated women with methadone during pregnancy. Abnormal fetal tests Notified that it occurs most often when the test is performed from 1 to 2 hours later a dose of methadone maintenance in late pregnancies compared to controls. Methadone does not produce teratogenic effects in rats or rats Rabbit models. Methadone produced teratogenic effects after large doses, in the hamster and the mouse. A study published in pregnant hamsters indicated that a single subcutaneous dose of methadone ranged from 31 to 185 mg/kg (the dose of 31 mg/kg is approximately 2 times the daily oral dose of humans 120 mg/day on a basis of mg/m2) on 8th day of gestation resulted in a decrease in the number of fetuses per litre and an increase in the percentage of fetuses exhibiting malformations described as exencephalia, craniosquisis and "vario other injuries." Most tested doses also resulted in maternity Death. In another study, a single subcutaneous dose of 22 to 24 mg/kg methadone (The estimated exposure was approximately at a human daily oral dose 120 mg/day on a mg/m2) basis administered on 9th day of gestation in mice also produced exencephalia in 11% of embryos. However, no effects were reported in oral doses of up to 40 mg/kg (estimated exposition) approximately 3 and 6 times, respectively, a human daily oral dose of 120 mg/day on a mg/m2) basis administered during 6 to 15 and 6 to 18, respectively. Published animal data have reported an increase in neonatal mortality in the descent of male rodents that were treated with previous methadone to mating. In these studies, female rodents were not treated with methadone, indicating paternally mediated toxicity of development. Specifically, Methadone administered to the male rat before pairing with methadone-naïve Women resulted in a decrease in weight gain after weaning. The male progenie demonstrated a weight reduction, while female progenie demonstrated Adrenal weight gain. Behavioral tests of these male and female progeny revealed significant differences in behavioral testing compared to control animals, suggesting that exposure to methadone can produce physiology and behavioral changes in progenie in this model. Other animal studies reported that opioid exposure including neuronal altered methadone development and behavior in the offspring. Methadone perinatal exposure in rats has been linked to alterations in learning capacity, motor activity, thermal regulation, nociceptive responses and drug sensitivity. Additional animal data show evidence for neurochemical changes in the brain of methadone treated seed, including changes to cholinergic, dopaminergic, noradrenergic and serotonergic systems. Studies showed that the treatment of male rat methadone for 21 to 32 days before pairing with methadone-nay females he did not produce any harm effects, suggesting that the protracted treatment of the male rat turned out in tolerance for development toxicities identified in progenie. Mechanical studies in this rat model suggests that the development effects of "parental" the methadone in the progenie seems to be due to the decrease in the production of testosterone. These animal data reflect reported clinical findings of testosterone decrease levels in human men in methadone for addiction to opioids and in men receiving chronic intraspinal opioids. Additional data have been issued indicating that treatment of male rat methadone (once a day for three consecutive days) increased embryocy and neonatal mortality. Review of uterine content mice fembras metadonously naive, raised to mice treated with methadone methadone treatment resulted in an increase in the rate of pre-implanted deaths in all postmetic states. Work and DeliveryMethadose is not for use in women during and immediately before work, where analgesics act shorter or other analgesic techniques are more appropriate [see ]. Opioid analgesics may prolong work temporarily reducing strength, duration and frequency Uterine contractions. However, these effects are not consistent and can be compensated by an increase in the rate of , which tends to shorten the job. INDICATIONS AND USAGEOpioids with mixed agonist properties should not be used for pain control during delivery in patients treated chronically with methadone as they may precipitate the acute withdrawal [see]. DROGA INTERACTIONS Opioids cross the placenta and can produce breaths depression and psychotic effects in neonates. Watch the neonates nearby whose mothers received opioid analgesics during childbirth to detect signs of respiratory depression. An opioid antagonist, such as, should be available for investment Opioid-induced respiratory depression in the .Nursing MothersMethadone is secreted in human milk. In the oral maternal case dose of 10 to 80 mg/day, methadone concentrations of 50 to 570 mcg/L in milk It has been reported that, in most samples, they were lower than mothers concentration of serum drugs in a stable state. Methadone levels in milk occur approximately 4 to 5 hours after an oral dose. Based on an average milk consumption of 150 ml/kg/day, a baby would consume approximately 17.4 mcg/kg/day, which is approximately 2-3 per cent of the oral maternal dose. Methadone has been detected in very low plasma concentrations in some babies whose Mothers were taking methadone. Cases of sedation and respiratory depression Babies exposed to methadone have been informed through breast milk. Precaution must be exercised when the methadone is given to a woman in nursing. Advise women who are being treated with methadone and who are breastfeeding or expressing a desire to breastfeed the presence of the methadone in human milk. Instructing nursing mothers how to identify the airways depression and sedation in your babies and when you need to contact your health care provider or seek immediate medical care. Breastfeeding infants mothers who use methadone should gradually tear themselves to prevent development withdrawal symptoms in the baby. Pediatrics UseMethadone safety, effectiveness and pharmacokinetics in pediatric patients under 18 years of age have not been established. Geriatric Use Clinical methadone studies did not include enough Numbers of subjects over 65 years old to determine if they respond differently compared to younger subjects. Other clinical experiences reported not It identified differences in responses between older and younger patients. In general, start elderly patients at the lower end of the dosage range, counts the highest rate of decrease in liver, kidney or heart function and concomitant disease or other drug therapy in geriatric patients. Almost. monitor older patients for signs of respiratory and central nervous system Depression. Neonatal Opioid Retreat SyndromeThe chronic maternal use of methadone during pregnancy can affect the fetus with subsequent withdrawal signs. Neonatal withdrawal syndrome presents as irritability, and abnormal sleep pattern, high Creole, vomiting, diarrhea and lack of weight gain. The beginning, duration and severity of neonatal withdrawal syndrome vary according to the drug use, duration of use, dose of last maternal use and elimination rate Newborn drug. Neonatal opioid withdrawal syndrome, unlike opioids Abtinence syndrome in adults, can be life-threatening and should be treated according to protocols developed by experts. Renal Impairment Methadone pharmacokinetics has not been extensively evaluated in patients with kidney failure. From the methadone without metabolizing and their metabolites are excreted in the urine to a varying degree, start these patients in lower doses and with longer dosing intervals and slowly titrate carefully monitoring the signs of the central and respiratory nervous system Depression. Hepatic Impairment Methadone has not been widely evaluated in patients with liver failure. Methadone is metabolized by liver paths; therefore, patients with liver disabilities may be at risk of increased systemic exposure to methadone after multiple dosing. Start these patients at lower doses and titrate slowly while carefully monitoring the signs of the airway and central nervous system depression. OVERDOSE Clinical symptoms Acute overdose is manifested by the airways depression, progress toward the stupor or coma, most restricted pupils, skeletal-muscle flaccidity, cold skin and clammy, and sometimes, and . In severe overdose, particularly by intravenous route, , collapse, cardiac arrest and death It happens. Overdose treatment In case of overdose, priorities are the re-establishment of a and protected and institutions of assistance or control if necessary. Use other support measures (including oxygen, vasopressors) in and as indicated. Cardiac Detention or arrhythmias will require advanced techniques. Opioid antagonists, like, are specific antidotes to respiratory depression resulting from opioid overdose. Opioid antagonists should not be given in the absence of clinically significant breathing or circulatory depression secondary to methadone overdose. Those agents should be be administered cautiously to patients who are known, or are suspected, depends physically on Methadose. In such cases, abrupt or complete Investment of opioid effects may precipitate acute withdrawal syndrome. Because the duration of the investment is expected to be less than the duration of the methadone action on Methadose, monitor carefully the patient until the spontaneous is re-established. Yeah. response to opioid antagonists is suboptimal or unsustained, additional antagonist should be given as indicated in the product's prescripts information. In an individual physically dependent on opioids, administration of an opioid receptor antagonist may precipitate an acute withdrawal. The severity of the withdrawal produced will depend on the degree of dependency and dose of the administered antagonist. If a decision is taken treat severe respiratory depression in the patient physically dependent, the administration of the antagonist must begin with care and by the degree with smaller doses than usual antagonist. CONTRAINDICATIONSMethodus is contraindicated in patients with: CLINE PHARMACOLOGYMechanism of ActionMethadone chloride is a muagonist; a synthetic analgesic with multiple actions qualitatively similar to those of , the most prominent of which involves compound organs and organs . The main therapeutic uses for methadone are for and for detoxification or maintenance in opioid addiction. The Methadone withdrawal syndrome, although qualitatively similar to that of morphine, differs in that the start is slower, the course is longer, and symptoms are less severe. Some data also indicate that methadone acts as in the N-methyl-D-aspartate (NMDA) receptor. The contribution of the NMDA receptor antagonism to the effectiveness of the methadone is unknown. Other NMDA receptor antagonists have proven to produce animal effects. Pharmacokinetics After oral administration bioavailability Methadone ranges between 36 to 100% and peak plasma concentrations are achieved between 1 and 7.5 hours. The proportionality of methadone pharmacokinetic dose is You don't know. However, after the administration of daily oral doses go from 10 to 10 225 mg, stable plasma concentrations ranged from 65 to 630 ng/m L and maximum concentrations ranged from 124 to 1255 ng/mL. Effect of food the bioavailability of methadone has not been evaluated. Methadone is a lipophilic medication and fixed status volume ranges from 1.0 to 8.0 L/kg. In plasma, methadone is predominantly tied to 1-acid (85% to 90%). Methadone is secret in , breast milk and plasma. Methadone is metabolized mainly by demethylation N to a inactive metabolite, 2-ethylidene-1,5-dimethyl-3,3- diphenylpyrrolidene(EDDP). Citocroma P450 enzymes, mainly CYP3A4, CYP2B6, and CYP2C19 and a minor extension CYP2C9 and CYP2D6, are responsible for converting methadone to EDDP and other inactive metabolites, which are mainly excreted in the urine. Methadone seems to be a substrate for glycoprotein P but its pharmacokinetics does not appear to be significantly altered in case of P-glycoprotein or inhibition. Excresion Methadone removal is mediated by extensive biotransformation, followed by renal and fecal excretion. Reports issued that after multiple doses administration the apparent plasma cleaning Methadone ranged from 1.4 to 126 L/h, and half terminal life (T1/2) highly variable and ranges from 8 to 59 hours in different studies. Methadone is a basic compound (pKa=9.2) and pH of the can altering its plasma disposition. Besides, since the methadone is lipophilic, it has It is known that they persist in the liver and other tissues. The slow release of the liver and other tissues may prolong the duration of the methadone action despite low plasma concentrations. Drug interactions Methana suffers liver N-demethylation by cytochrome P450 (CYP) isoforms, mainly CYP3A4, CYP2B6, CYP2C19, and a minor extension by CYP2C9 and CYP2D6. Methadone co-management with CYP inductors can lead to greater speed and potential to reduce the effects of methadone, while administration with CYP inhibitors can reduce metabolism and enhance the effects of methadone. Although drugs like efavirenz, nelfinavir, nevirapina, ritonavir, lopinavir+ritonavir combination are known to inhibit some CYPs, it is shown to reduce plasma levels methadone, possibly due to the induction activity of CYP [see ]. Therefore, medications administered concomitantly with methadone should be evaluated for interaction potential; clinicians are advised to evaluate response to drug therapy. DROGA INTERACTIONS The following interactions were reported between medicines after co-administration of methadone with known inducers of P450 cytochrome enzymes: Rifampin: In patients well stabilized in methadone, the concomitant management of rifampina led to a marked reduction in the methadone seero levels and a concurrent appearance of . Rifampin:Phenytoin: In a pharmacokinetic study with patients about methadone , phenitoin administration (250 mg twice a day initially for 1 day followed by 300 mg daily for 3 to 4 days) resulted in a about 50% reduction in exposure to methadone and withdrawal symptoms It happened simultaneously. After the interruption of phenitoin, the incidence withdrawal symptoms decreased and exposure to methadone increased to a level comparable to the previous administration of phenitoin. Fenitoin: St. John's Wort, Fenobarbital, Carbamazepine: Administration Methadone with other CYP3A4 inducers may result in withdrawal symptoms. The herb of San Juan, Fenobarbital, Carbamazepina: Since the metabolism of the methadone is mediated mainly by CYP3A4 isozyme, coadministration of drugs that inhibit CYP3A4 activity may causes methadone decrease. Voriconazole: Repeated oral administration dose voriconazole (400 mg every 12 hours for 1 day, then 200 mg every 12 hours for 4 days) increased the maximum concentration of plasma (Cmax) and AUC of (R)-methadone by 31 per cent and 47 per cent, respectively, in subjects receiving maintenance of methadone dose (30 to 100 mg a day. The Cmax and AUC of (S)-methadone increased by 65% and 103%, respectively. Methadone plasma concentrations have increased associated with toxicity including QT prolongation. Frequent monitoring adverse events and toxicity related to methadone is recommended during co-administration. Methadone dose may be reduced [see ].Voriconazole: Although antiretroviral drugs such as efavirenz, nelfinavir, nevirapina, ritonavir, webprevir, lopinavir+ritonavir combination are known inhibit some CYPs, it is shown that they reduce the plasma levels of the methadone, possibly due to CYP induction activity. Abacavir, amprenavir, darunavir+ritonavir, efavirenz, nelfinavir, nevirapine, ritonavir, webprevir, lopinavir+ritonavir, saquinavir +ritonavir, tipranvir+ritonavir combination: Coadministration of these antiretrovirals agents resulted in an increase in the cleaning or decrease in the plasma levels of the methadone [see ]. Abacavir, amprenavir, darunavir+ritonavir, efavirenz, nelfinavir, nevirapine, ritonavir, webprevir, lopinavir+ritonavir, saquinavir +ritonavir, tipranvir+ritonavir combination: Coadministration of these antiretrovirals agents resulted in an increase in the cleaning or decrease in the plasma levels of the methadone [see ]. Didanosine and Stavudine: Methadone decreased the AUC and maximum levels of didanosine and stavudine, with a more significant decrease by didanosine. The disposition of the methadone was not substantially altered [see ].Didanosine and Stavudine:Zidovudine: Methadone increased the AUC which could result in toxic effects [see ]. Zidovudine: INTERACTIONSPATIENT INFORMATIONMEDICATION GUIDEMEDICATION GUIDEMethadoseTM Oral tablets (methadone chloride tablets USP)MethadoseTM Methadose is:Methadose is:Important information about Methadose: Important information about Methadose: Do not take Methadose if you have: Do not take Methadose if you have: Before taking Methadose, tell your health care provider if you have: You have a story of: Before taking Methadose, tell your health care provider if You have a history of: Tell your health care provider if you are: Tell your health care provider if you are: pregnant or plan to become pregnant. When taking Methadose: When taking Methadose:Call your health care provider if the dose you are taking Don't control your pain. Do not stop taking Methadose without talking to your medical care provider. While I was taking Methods No:While you are taking Methadose No: Possible side effects of Methadose are: Possible side effects of Methadose are: Get emergency medical help if you have: Get emergency medical help if you have: These are not all possible side effects of Methadose. Call your doctor to advise you about side effects. You may report side effects a la FDA at 1-800-FDA-1088. For more information go to dailymed.nlm.nih.gov This Medicines Guide has been approved by the US. Drug administration. From Food and Drug Administration Reporting ProblemsYou are encouraged to report negative side effects of prescription drugs to the FDA. Visit the website or call 1-800-FDA-1088. Healthcare solutions for our sponsorsQuick, easy identification tool, pill identification Drug interaction tool Drug interaction control Pharmacy Locater tool Including 24 hours, Dolophine Pharmacies around, white, 54 210round printed, white, 54 142 printed, white, 54 142 rectangular, white, 57 55 printed, white .
Methadone, Methadone OralMethadone is a prescription drug. It is an opioid, which makes it a . This means that this medicine has a risk of misuse and can cause dependency. Methadone comes as an oral tablet, oral dispersible tablet (table that can be dissolved in liquid), oral concentrate solution and oral solution. Methadone also comes intravenous (IV), which is only given by a health care provider. Methadone is also available as the Methadose brand medication, which comes in an oral soluble tablet. Methadone oral tablet is used to administer moderate to intense pain. It is only given when other short or non-opioid pain medications do not work for you or if you cannot tolerate them. Methadone is also used to administer drug addiction. If you have an addiction to another opioid, your doctor may give you methadone to prevent you from having serious withdrawal symptoms. How it Works Methadone belongs to a class of drugs called (narcotics). A class of drugs is a group of drugs that work similarly. These drugs are often used to treat similar conditions. Methadone works on pain receptors in her body. Reduce the pain you feel. Methadone can also replace another opioid drug you have an addiction to. This will prevent you from experiencing severe withdrawal symptoms. This drug can make you very sleepy. You should not drive, use machinery, or do other activities that require alert after you have taken this medication. This drug can make you very sleepy. You should not drive, use machinery, or do other activities that require alert after you have taken this medication. Methadone may cause mild or severe side effects. The following list contains some of the key side effects that can occur when taking methadone. This list does not include all possible side effects. For more information on the possible side effects of methadone, or advice on how to deal with a worrying side effect, talk to your doctor or pharmacist. Most common side effects Most common side effects of methadone may include: If these side effects are mild, they may disappear within a few days or a couple of weeks. If you are more severe or do not go away, talk to your doctor or pharmacist. Serious Side Effects Call your doctor immediately if you have serious side effects. Call 911 if your symptoms feel life threatening or if you think you're having a medical emergency. Serious side effects and symptoms may include the following: The dose of methadone prescribed by your doctor will depend on several factors. These include:Typically, your doctor will start you at a low dose and adjust it over time to reach the dose that is right for you. Ultimately, they will prescribe the smallest dose that provides the desired effect. The following information describes the doses commonly used or recommended. However, be sure to take the dose prescribed by your doctor. Your doctor will determine the best dose to meet your needs. Generic Ways and Fortresses: Methadone Brand: Methadose Dosage for moderate to severe short-term pain Adult dose (ages 18 to 64 years old) Infant dose (age 0-17 years) The safety and effectiveness of this drug has not been established in children. It should not be used in children under 18 years of age. Upper dose (ages 65 and older)Your kidneys may not work as they used to. This can make your body process drugs more slowly. As a result, a larger amount of medication remains in your body for a longer time. This increases the risk of side effects. Dosage of opioid addiction Adult dose (ages 18 to 64 years old) Infant dose (age 0-17 years old) The safety and effectiveness of this drug has not been established in children. It should not be used in children under 18 years of age. Upper dose (ages 65 and older)Your kidneys may not work as they used to. This can make your body process drugs more slowly. As a result, a larger amount of medication remains in your body for a longer time. This increases the risk of side effects. Dosage for the maintenance of opioid addiction Adult dose (ages 18 to 64 years) The standard dose ranges from 80–120 mg per day. Your doctor will determine a proper dose for you. Infant dose (age 0-17) The safety and effectiveness of this drug has not been established in children. It should not be used in children under 18 years of age. Upper dose (ages 65 and older)Your kidneys may not work as they used to. This can make your body process drugs more slowly. As a result, a larger amount of medication remains in your body for a longer time. This increases the risk of side effects. Important Warning Do not crush, dissolve, snort or inject oral methadone tablets because this can cause you to overdose. This can be fatal. When to Call Your Doctor Methadone oral tablet is used for short-term treatment. It comes with serious risks if you don't take it as prescribed. If you stop taking the medication suddenly or don't take it at all: Your pain may not be controlled and may go through opioid removal. The withdrawal symptoms include: If you lose dose or do not take the medication in the schedule: Your medication may not work too or may stop working completely. You may also experience withdrawal symptoms. If you take too much: You could have dangerous levels of the drug in your body. Symptoms of an overdose of this medication may include: If you think you have taken too much of this medication, call your doctor or local poison control center. If your symptoms are severe, call 911 or go to the nearest emergency room immediately. What to do if you miss a dose: If you are taking this medicine to treat pain: Do not take more of your prescribed dose in 24 hours. If you take this pain medication and lose a dose, take it as soon as possible. Then take the following dose from 8 to 12 hours later, as indicated by your doctor. If it is almost time for your next dose, skip the missed dose and return to your regular dosing schedule. If you are taking this medication for detoxification and addiction maintenance: Take the following dose the next day as scheduled. Do not take additional doses. Taking more of the prescribed dose can cause you to overdose because this medication accumulates in your body over time. How to know if the medication is working: Should have decreased pain or withdrawal symptoms should be gone. This drug comes with several warnings. FDA Warnings Somnolence Alert This drug can make you very sleepy. You should not drive, use machinery, or do other activities that require alert after you have taken this medication. Allergy alert Methadone can cause a severe allergic reaction. Symptoms may include: If you develop these symptoms, call 911 or go to the nearest emergency room. Do not take this medication again if you have ever had an allergic reaction. Taking it again could be fatal (by death). Alcohol interaction alert The use of alcohol-containing beverages may increase your risk of sedation, slow breathing, coma (which is unconscious for a long time), and methadone death. If you drink alcohol, talk to your doctor. You may need to be monitored by low blood pressure, respiratory problems and sedation. Warnings for people with certain health conditions For people with kidney problems: If you have kidney problems or a history of kidney disease, you may not be able to clean this medication properly from your body. This can increase methadone levels in your body and cause more side effects. Your doctor should monitor you closely if you take this medication. For people with liver problems: If you have liver problems or history of liver disease, you may not be able to process this medication well. This can increase methadone levels in your body and cause more side effects. Your doctor should monitor you closely if you take this medication. For people with respiratory problems: This medicine can cause respiratory problems. It can also worsen the breathing problems you already have. This can be fatal (for death). If you have breathing problems, severe asthma or have an asthma attack, you should talk to your doctor about whether this medication is safe for you. For people with gastrointestinal obstruction (GI): This medicine can cause constipation and increase the risk of GI obstruction. If you have a GI obstruction history or have one, you should talk to your doctor about whether this medication is safe for you. If you have a paralytic ileum (lack of muscle tone in your intestines that may cause obstruction of GI), you should not take this medication. For people with seizures: This medicine can cause more seizures in people with epilepsy. If your seizure control gets worse while taking this medication, call your doctor. For people with head injury: This medicine can cause greater pressure on your brain. This can increase your risk of complications or cause death. If you have had a recent head injury, you increase your risk of breathing methadone problems. Talk to your doctor about whether this medication is safe for you. Warnings for other groups Methadone may interact with several other medicines. Different interactions can cause different effects. For example, some may interfere with how well a medication works, while others may cause higher side effects. Below is a list of medicines that can interact with methadone. This list does not contain all medicines that can interact with X medication. Before taking methadone, be sure to tell your doctor and pharmacist about all prescriptions, free sale and other medicines you take. Also tell them about any vitamins, herbs and supplements you use. Sharing this information can help you avoid possible interactions. If you have questions about drug interactions that may affect you, ask your doctor or pharmacist. Drugs you shouldn't use with methadone Do not take the following methadone drugs. Doing it can cause dangerous effects on your body. Interactions that increase the risk of side effects Interactions that can make your drugs less effective When methadone is used with certain medications, it may not work too to treat your condition. This is because the amount of methadone in your body can be decreased. Examples of these drugs are: Please consider these considerations if your doctor prescribes methadone. GeneralStorageFill A prescription for this medication is not revigilable. You or your pharmacy will have to contact your doctor to get a new prescription if you need this stuffed medication. TravelWhen you travel with your medication: Autogestion Do not swallow the dispersible tablet before it has dissolved in a liquid. You should mix it with 3 to 4 ounces (90 to 120 milliliters) of water or citrus juice before taking it. It takes a minute to blend in. Clinical Surveillance You and your doctor should monitor certain health problems. This can help make sure you stay safe while taking this medication. These issues include: Prior Authorization There are restrictions on the dispensing of methadone for detoxification or maintenance programs. Not all pharmacies can dispense this medication for detoxification and maintenance. Talk to your doctor about where you can get this medication. There are other medicines available to treat your condition. Some may be more suitable for you than others. Talk to your doctor about other medication options that can work for you. Disclaimer: The health line has done everything possible to ensure that all information is in fact correct, complete and updated. However, this article should not be used as a substitute for the knowledge and experience of a graduated health professional. You should always consult your doctor or other health care professional before taking any medication. The drug information contained in this document is subject to change and is not intended to cover all uses, addresses, precautions, warnings, drug interactions, allergic reactions or possible adverse effects. The absence of warnings or other information for a given medication does not indicate that the combination of drugs or drugs is safe, effective or appropriate for all patients or all specific uses. Last medical review on October 13, 2020Read this following
Methadone Hydrochloride Oral Tablet 10Mg Drug Medication Dosage Information
Methadone hydrochloride Pill Images - What does Methadone hydrochloride look like? - Drugs.com
What is Methadone? - GoodRx
How Do I Identify Methadone? | Michael's House Treatment Centers
methadone (oral/injection) | Michigan Medicine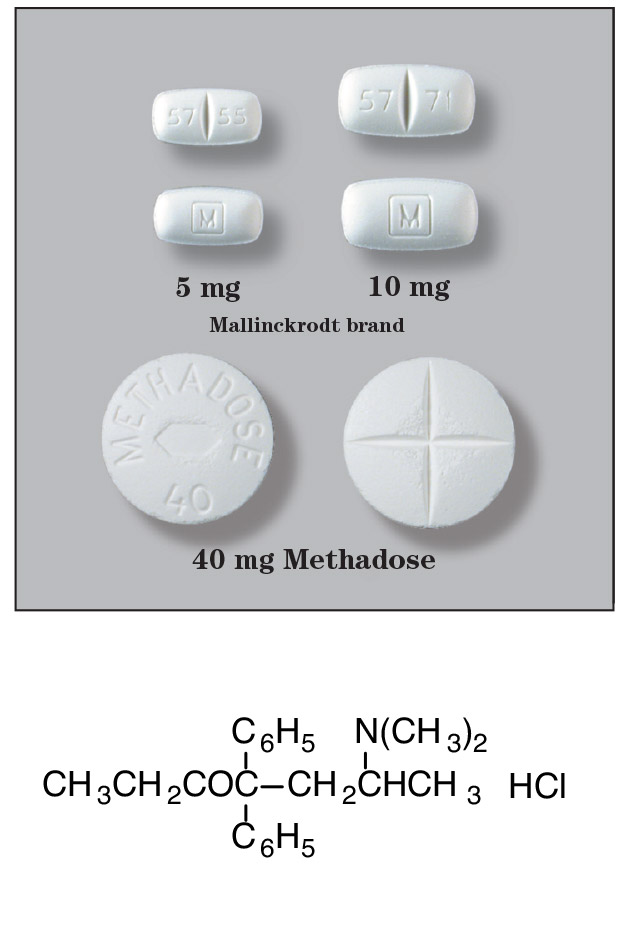
Methadone – Sigler Drug Cards
What is Methadone? - GoodRx
T293 Pill Images (White / Round)
Methadone: Uses, How To Identify & Addictive Qualities | The Recovery Village Palm Beach at Baptist Health
Methadone hydrochloride Pill Images - What does Methadone hydrochloride look like? - Drugs.com
Methadone 10 Mg Tablets - Drone Fest
Methadone Addiction and Abuse - Understanding a Methadone Addiction
Methadone Hydrochloride 10mg ASC 116 | Prodex Pharma
Buy Online Methadone 10 Mg Online | Buy Methadone Online
buy methadone tablets overnight delivery no prescription cheap
Whats left of my Methadone Rx after getting transd to Subutex..10mg methadone tablets : opiates
Methadone HCl Tablets 10mg Bottle 100/Bt - Henry Schein Special Markets
Methadone Tablets - Opiate Addiction & Treatment Resource
Buy Methadone Online - Buy Methadone Online Without Prescription
Roxane Laboratories 00054855324 - McKesson Medical-Surgical
Buy Methadone 10mg online - usadiscreetpharma.over-blog.com
buy methadone online
Methadone Hydrochloride Oral Tablet 10Mg Drug Medication Dosage Information
Signs and Symptoms of Methadone Abuse
Methadose Dosage Guide - Drugs.com
Buy Methadone 10mg – Dr. Mark Pharmaceuticals
Methadone Identification - Opiate Addiction & Treatment Resource
methadone (oral/injection) | Michigan Medicine
METHADONE HYDROCHLORIDE TABLETS USP 5 mg, 10 mgCIIRx only
Buy Methadone Hydrochloride 10mg Online | First Trust Euro Pharmacy
CrimLaw: Abused Drugs
Methadone - Meds Consulting
Methadone 40mg buy Online | No required Prescription
Methadone hydrochloride Pill Images - What does Methadone hydrochloride look like? - Drugs.com
Pill Finder: M 57 71 White Rectangle - Medicine.com
Mallinckrodt 00406577101 - McKesson Medical-Surgical
F.D.A. Rejects Mandatory Training in Painkillers for Doctors - The New York Times
Buy Methadone Tablets 10mg USA, Buy Methadone 10mg UK.
Buy Methadone Online ⋆ CIALISPHARMA ⋆ Methadone Tablets
 54 142 Pill Images (White / Round)
54 142 Pill Images (White / Round)

































Posting Komentar untuk "methadone pills 10 mg"