l carnitine for hyperthyroidism
 The Best Natural Supplements for Hyperthyroidism - ThyroMate
The Best Natural Supplements for Hyperthyroidism - ThyroMateArticle Contents Utility of l-Carnitine, A Naturally Occurring Peripheral Antagonist of Thyroid Hormone Action, in Iatrogenic Hyperthyroidism: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial CiteSalvatore Benven, Rosaria M. Ruggeri, Antonia Russo, Daniela Lapa, Alfredo Campenni, Francesco Trimarchi, Utility of l-Carnitine, A Naturally Occurring Peripheral Antagonist of Thyroid Hormone Action, in Iatrogenic Hyperthyroidism: A Randomized, Double-Blind, Placebo-Control Clinical94 Trial This conclusion was corroborated by our recent observation that carnitine inhibits the entry of thyroid hormone in the nucleus of hepatocytes, neurons and fibroblasts. In the randomized, double-blind, placebo-controlled trial of 6 months reported here, we evaluated whether 2 or 4 g/d of oral carnitine was able to reverse and prevent/minimise nine symptoms related to hyperthyroidism. We also evaluate changes in nine biochemical parameters sensitive to thyroid hormone and vertebral and hip mineral density (bone mineral density). Five groups of 10 subjects were randomly assigned to each 50 women with a fixed dose of I-T4 for 6 months. Group 0 placebo associated for 6 months; groups A2 and A4 began to associate placebo (first bimester), placebo replaced with carnitine 2 or 4 g/d (second bimester), and then returned to the association with placebo. The B2 and B4 groups began to associate carnitine of 2 and 4 g/d for the first two bimesters, and then replaced carnitine with placebo (third bimestre). The symptoms and biochemical parameters worsened in group 0. In group A, the symptoms and biochemical parameters worsened during the first quarter, returned to the base or increased minimally during the second bimester (except osteocalcin and OH urinary proline) and increased again in the third quarter. In group B, the symptoms and biochemical parameters (except osteocalcin and urinary OH-proline) did not get worse or improved in the first 4 months; they tended to get worse in the third bimester. Both in groups A and B, the two doses of carnitine were equally effective. At the end of the test, bone mineral density tended to increase in groups B and A (B mento A). In conclusion, carnitine is effective both for investment and to prevent symptoms of hyperthyroidism and has a beneficial effect on bone mineralization. Because hyperthyroidism exhausts carnitine body deposits and because carnitine does not have toxicity, teratogenicity, contraindications and interactions with drugs, carnitine may be of clinical use. L-CARNITINE IS A quaternary amine (hydroxy-γ-trimetilammonium butyrate) that is omnipresent in biological fluids and tissues of mammals, where it plays an important role in energy metabolism (–). Primary and secondary carnitine deficiencies, including heart carnitine depletion associated with coronary heart disease and heart failure, are the therapeutic indications for carnitine (). Oral doses usually range from 1 to 4 g per day (). Ancient animal studies showed that carnitine is capable of contrasting thyroid hormonal changes associated with the metamorphosis of tadpoles and the nitrogen balance of rats (–). The same group of authors () also showed that serum and hepatic concentrations of aminotransferase alanine (ALT) and aminotransferase aspartate (AST) increased in rats treated with T4, but decreased in rats treated with carnitine. These studies were followed by unpublished studies on a small number of torotoxic patients, who were treated only with oral carnitine from 1 to 3 g per day for a few weeks (–). Although there was a lack of quantification of symptomatology and statistical analysis, the authors (–) reported a definitive improvement in symptomatology from the second week of treatment; goiter size, thyroid [131]I consumption, ophthalmopathy and iodine with serum proteins were unaltered (–). Carnitine was therefore regarded as a peripheral antagonist of thyroid hormone action, not a function inhibitor of the thyroid gland (–). Considering that so far we do not have an ideal antagonist of thyroid hormonal action, it is surprising that no further studies have been conducted. We have developed an interest in carnitine and have found that it inhibits the entry of thyroid hormone in the nucleus of human and animal cells (fibroblasts, hepatocytes, neurons), thus explaining peripheral antagonism. Based on this experimental evidence (), we wanted to perform a controlled trial to test the clinical use of carnitine. Patients & MethodsGeneralities We tested the potential benefits of carnitine in adult women (e.g., the vast majority of thyroid patients) under doses of TSH-supressive l-throoxine for benign nodular goiter. We prefer these patients to patients with Graves disease for several reasons, including additional medications and/or non-medical therapy modalities that these last patients might require, thus complicating the study. In addition, with our protocol we could evaluate a double carnitine effect, namely, whether it was able to invest and prevent hyperthyroidism. In pilot studies on humans, we note that carnitine does not antagonize the negative physiological feedback of thyroid hormones in TSH secretion. Study Design (Protocol) This randomized, double-blind, placebo-controlled trial with placebo-carnitine crossing had been approved by the Ethics Committee of our University. Criteria for entry, after the signature of the informed consent form, were: 1) to be in good health, based on thorough physical examination and routine clinical chemistry; 2) not previous use of thyroid and carnitine hormones; and 3) not use other medications. The patients were randomly assigned to three main groups of 10 people each: 0 (zero, which means there is no carnitine at any time), A and B. All patients, who were eutiroids at the base, received 2.0–2.4 μg per kg of body weight per day () orals l-tiroxine (Eutirox; Bracco, Milan, Italy), which was taken 2–3 h before breakfast to maximize intestinal absorption (). Although it is not necessary in a cross study, group 0 served as a substitute group, that is, it served to show what might have happened to patients in group A and B if it is treated with l-tiroxin alone. The A and B groups consisted of two subgroups each (A2, A4, B2, B4), based on the daily oral dose (2 or 4 g, when indicated) of l-carnitine; clearly, the two subgroups served to evaluate the possible dose-dependence of carnitine effects. l-carnitine (Carniteno, incoming liquid vials of 1 or 2 g; Sigma-Tau, Pomezia, Italy) was taken twice a day (1 jar after lunch, 1 jar after dinner). In group A, l-thyroxine was associated with placebo from 1 to 60 or with carnitine during d 61 to 120 or again with placebo during d 121 to 180. In group B, l-thyroxine was associated to carnitine during d 1 to 120 or placebo during d 121–180. We decided to administer carnitine for 4 months in group B to have consistent evidence of its effectiveness (4 months, not 2 months). The day 0, 30, 60, 90, 120, 150 and 180 (± 1 day) was evaluated clinically and biochemically to the 50 patients (see below). In addition to the tablet count, the compliance of the l-tiroxine was evaluated by measuring thyroid hormones free of serum (FT3, FT4) and TSH (which had to be ≤0.01 mU/l) in all visits. The three hormones were measured with the Boerhinger electrochemiluminescent trial (Mannheim, Germany). The corresponding variation coefficients (CVs) are 2.8%, 2.5% and 2.2% (intra-assay) and 3.9%, 3.8% and 3.3% (assay). In addition to counting the vial, the performance of the carnitine was evaluated by measuring the urinary excretion of 24h of carnitine () (courtesy of the Dres. A. Toscano and M. d'Agennouz, Clinica Neurologica 2, University of Messina), with patients who are not aware of this particular range of urine collection. shows compliance with the two drugs and, at the same time, the homogeneity of the five groups in terms of serum hormones. Changes in serum FT3, FT4, TSH and 24h carnitine urinary excretion upon entry and during the three biomesters of the clinical trial in the five study groups. Not to complicate the figure, there are no bars. Note the overlap of the hormonal profiles between groups, and the greatest excretion of carnitine in the treated groups of 4 g/d (A4, B4) vs. the 2 g/d-treated (A2, B2). Changes in serum FT3, FT4, TSH and 24h carnitine urinary excretion upon entry and during the three biomesters of the clinical trial in the five study groups. Not to complicate the figure, there are no bars. Note the overlap of the hormonal profiles between groups, and the greatest excretion of carnitine in the treated groups of 4 g/d (A4, B4) vs. the 2 g/d-treated (A2, B2). The average age (±se) in each group was not statistically different from the age of the other groups: 40.0 ± 2.8 (group 0), 48.3 ± 4.2 (A2), 43.4 ± 5.8 (A4), 42.2 ± 2.8 (B2), and 40.1 ± 4.9 yr (B4). Evaluation of the symptomatology Other authors (cf.) have discussed the unnecessary complications of certain "clinical indexes" in favor of simple clinical rules. Clinical indices are numerous (e.g. Refs. –), since none is satisfactory. The prototype of these indexes is the Crooks index (), which is a unique number resulting from a score system based on the presence or absence of some symptoms and signs. Current symptoms are assigned different scores (e.g. dyspnea +1, palpitations +2, weight reduction +3), which are fixed, so that changes in their severity cannot be quantified. Based on our experience in hundreds of naturally hyperthyroid patients under antithyroid therapy and thousands of patients under TSH suppressive therapy, we have built the 5-point scale presented to quantify symptoms. Compared to the Crooks index (), we have omitted sweat, because the inscription and follow-up of different patients occurred in different stations, and have added reflexes of insomnia and knee. In 100 adult hyperthyroid women with FT4 had been measured with the same trial used in the present study, the correlation of our serum score system FT4 was 0.41 (P Standardized Syntomatology AssessmentScore . Symptomology . Subjective . Objective . Tremors . Reflexes . 1 Common Absente 2 Occasional but disturbing when present Very well Barely brisk 3 Frequent; disturbing Fine Moderately brisk 4 Most frequent; more disturbing Mildly gross Markedly brisk 5 Constant, intolerable Deep; tremor of hands Extremely exaggerated, polyphase scores . Symptomology . Subjective . Objective . Tremors . Reflexes . 1 Common Absente 2 Occasional but disturbing when present Very well Barely brisk 3 Frequent; disturbing Fine Moderately brisk 4 Most frequent; more disturbing Mildly gross Markedly brisk 5 Constant, intolerable Very disgusting; tremor of the hands Extremely exaggerated, polyphasic Asthenia, dyspnea, insomnia, nerves. Hiding for tremors almost coincides with the scale of 5 points from 0 to 4 of Klein et al. ( ): 0, absent; 1, barely perceptible; 2, easily demonstrated in examination; 3, marked; 4, hands shake excessively. We evaluate the knee reflections in the patient sitting with legs hanging loosely from the edge of the test board. With the distance-foot examiner given from the rest legs, we define the normal legs (score 1) that advance forward from 5 to 10 cm after the percussion of the patellar tendon, and the three degrees of briskness as legs that advance forward from 11 to 20 cm (score 2), 21 to 30 cm (score 3), and 31 to 40 cm (score 4). The other two objective parameters [heart rate (in beats per min) and body weight (in kg)] were evaluated by heart auscultation and weighing. All patients were weighed on the same scale. Standardized Evaluation of Syntomatology Score . Symptomology . Subjective . Objective . Tremors . Reflexes . 1 Common Absente 2 Occasional but disturbing when present Very well Barely brisk 3 Frequent; disturbing Fine Moderately brisk 4 Most frequent; more disturbing Mildly gross Markedly brisk 5 Constant, intolerable Deep; tremor of hands Extremely exaggerated, polyphase scores . Symptomology . Subjective . Objective . Tremors . Reflexes . 1 Common Absente 2 Occasional but disturbing when present Very well Barely brisk 3 Frequent; disturbing Fine Moderately brisk 4 Most frequent; more disturbing Mildly gross Markedly brisk 5 Constant, intolerable Very disgusting; tremor of the hands Extremely exaggerated, polyphasic Asthenia, dyspnea, insomnia, nerves. Hiding for tremors almost coincides with the scale of 5 points from 0 to 4 of Klein et al. ( ): 0, absent; 1, barely perceptible; 2, easily demonstrated in examination; 3, marked; 4, hands shake excessively. We evaluate the knee reflections in the patient sitting with legs hanging loosely from the edge of the test board. With the distance-foot examiner given from the rest legs, we define the normal legs (score 1) that advance forward from 5 to 10 cm after the percussion of the patellar tendon, and the three degrees of briskness as legs that advance forward from 11 to 20 cm (score 2), 21 to 30 cm (score 3), and 31 to 40 cm (score 4). The other two objective parameters [heart rate (in beats per min) and body weight (in kg)] were evaluated by heart auscultation and weighing. All patients were weighed on the same scale. Patients were asked to follow up on changes in the frequency, intensity and tolerability of symptoms. In each monthly visit, patients were interviewed and examined clinically. The clinical examination was performed by two authors of this study, but separately. A third author served to resolve disagreements, but this intervention was necessary in only 4 of 350 times (50 patients × 7 visits), and occurred in control visits that were not particularly relevant (fifth or sixth month). Biochemistry Assessment Although oral doses of up to 15 mg l-carnitine per day are well tolerated (), however, we monitor routine blood chemistry and urinalysis monthly. Among the blood parameters, some are regulated by thyroid hormones. ALT, AST,γ -glutamiltransferase (GGT), and alkaline phosphatase are regulated, while phosphokinase creatine and cholesterol are deregulated (). All of these parameters were measured with the IL 900 Autoanalyzer (Instrumental Ship, Milan, Italy) using colorimetric kits that have intra-assay CVs less than 4% and interassay CV less than 6%. For the purposes of this study, sera was stored in −20 C until the trial was completed. Then, each parameter in the 350 samples (7 samples/patient × 10 patients/group × 5 groups) was measured in the same race. Also, after the end of the trial and in a single race, four additional parameters were measured, all regulated by thyroid hormones: globulin of sexual hormonal union (SHBG), ferritin, osteocalcin and urinary excretion of hydroxyprolin (OH-P) (). Serum SHBG and osteocalcin were measured with the imunoradiometric essay of Radim (Pomezia, Italy) and CIS (Gif-sur-Yvette, France), respectively; ferritin with the quimiluminescent essay of Diagnostic Products (Los Angeles, CA); and OH-P with the high-performance liquid chromatography kit of Bio-Rad Laboratories, Inc. The corresponding intra-assay CV was 5.1%, 3.8%, 5.2% and 5.2%, while the interassay CV was 5.2%, 4.7%, 8.2% and 6.6%. Bone Density Assessment Thyroid hormones have a dual effect on the bone: stimulation of osteoblasts, with the subsequent increase of bone formation markers such as serum osteocalcin and stimulation of osteoclasts, with the subsequent increase of bone resorption markers such as urinary OH-P (). Because of this and due to the controversy surrounding the relationship between thyroid hormone treatment in the bone (), we thought it was of interest to complement the biochemical measurements of serum osteocalcin and urinary OH-P with a clinically relevant parameter: bone mineral density (BMD). BMD (g/cm2) of the lumbar spine (L2–L4) and the left femur was measured at the base and end of the double-energy X-ray absorption test using a Hologic QDR-2000 instrument (Waltham, MA). The second or post-treatment DMO cannot always be scheduled on the 180th day (± 1 day) (viz. on the same day of the last visit and the collection of blood and urine). For this reason, two to four patients in each group did not report themselves to the second MMD. Accordingly, the two MMD measurements are available for six to eight subjects per group. Adverse events Undesirable clinical symptoms reported by patients were recorded in each visit from d 1, based on direct clinical questioning. In addition to the vital test of signs, physical and chemical examinations of blood and urine were performed in all visits. Statistical Analysis The data is presented as mean ± se; the 95% confidence interval (CI) is also given for changes. The media differences were analyzed by ANOVA. The level of statistical importance was set at P below 0.05. ResultsChanges in serum FT3, FT4, TSH and 24h urinary excretion of carnitine at the entrance and during the trial are reported in . As expected, the 50 patients were confirmed to be eutiroids at the baseline by the thyroid function tests. Differences between groups relating to FT3, FT4, and TSH are not statistically significant (P ≤ 0.05). The FT3 serum, FT4, and TSH at the entrance oscillated 4.1 ± 0.2 (group B2) to 4.5 ± 0.2 pmol/l (group 0), 14,8 ± 0.7 (group A2) to 16.5 ± 0.1 pmol/l (group A4) and 1.4 ± 0.4 (group B2) to 1.9 ± 0.5 mU/l (group A2), respectively, In the visit of 1 month and then, in the five groups the serum FT3 was in the upper-normal range, the serum FT4 was above the upper normal limit, and the serum TSH was systematically removed (). Again, there were no statistical differences between groups in the three hormonal levels (P ≥ 0.05) throughout the trial. The urinary excretion of carnitine at the entrance ranged from 206 ± 37 (group 0) to 251 ± 4.1 μmol/24 h (group B4) (P 0.05). The urinary carnitine reached the maximum expected (viz. months 3 and 4 in both groups A2 and A4; months 1 to 4 in both groups B2 and B4), and with the expected gradient (A4 √≥ A2, and B4 ≥ B2) (, demonstrating that patients were compatible with carnitine administration. Reversal effect of carnitineSyptomatology. Modifications of clinical parameters in groups 0, A2, and A4 are shown in the three top panels of -4. The first eight parameters are positively regulated by thyroid hormones; therefore, the increase indicates worsening. The body weight is regulated negatively; therefore, the deterioration is indicated by a decrease in kilograms. During the first 2 months of the symptoms/signs of the trial worsened to a similar extent in the three groups. However, the profile of the changes differed during the next 2 months in group 0 (simtoms increased) vs. groups A (symptoms decreased). When the placebo was reintroduced instead of carnitine (fifth and sixth month of therapy), the symptoms worsened (or tended to) in A2 and A4 groups. In general, during the 6 months of the trial, the intensity of each symptom/sign in the A2 and A4 groups was statistically different from group 0 (P 0.05). Variations (medium ± se) of the three clinical parameters indicated in the five patient groups during the duration of 6 months of the trial. Variations of the remaining six clinical parameters and 4. All patients took doses of TSH-suppressive of l-tiroxine from 1 to 180, which was always associated with placebo in the 10 patients of group 0. In the group A2 (n = 10) and A4 (n = 10) patients, who were enrolled to evaluate whether 2-4 g/d of carnitine l oral were able to reverse iatrogenic hyperthyroidism, placebo was associated during the months 1, 2, 5 and 6, and carnitine during the months 3 and 4 (▪). In group B2 (n = 10) and B4 (n = 10) patients, who were enrolled to evaluate whether 2-4 g/d carnitine l oral were able to prevent iatrogenic hyperthyroidism, placebo was associated during the months 5 and 6, and carnitine during the months 1 to 4 (▪). For each symptom, all data points from 0 to 6 months in group 0 are compared to all data points in A2 and A4 or B2 and B4 groups by ANOVA, and the resulting P value is shown. The relevant percentage changes in the severity of each symptom are summarized and contrasted between the groups in and 11. For more details on the evaluation of symptomatology, see and text (Patients and Methods). Variations (medium ± se) of the three clinical parameters indicated in the five patient groups during the duration of 6 months of the trial. Variations of the remaining six clinical parameters and 4. All patients took doses of TSH-suppressive of l-tiroxine from 1 to 180, which was always associated with placebo in the 10 patients of group 0. In the group A2 (n = 10) and A4 (n = 10) patients, who were enrolled to evaluate whether 2-4 g/d of carnitine l oral were able to reverse iatrogenic hyperthyroidism, placebo was associated during the months 1, 2, 5 and 6, and carnitine during the months 3 and 4 (▪). In group B2 (n = 10) and B4 (n = 10) patients, who were enrolled to evaluate whether 2-4 g/d carnitine l oral were able to prevent iatrogenic hyperthyroidism, placebo was associated during the months 5 and 6, and carnitine during the months 1 to 4 (▪). For each symptom, all data points from 0 to 6 months in group 0 are compared to all data points in A2 and A4 or B2 and B4 groups by ANOVA, and the resulting P value is shown. The relevant percentage changes in the severity of each symptom are summarized and contrasted between the groups in and 11. For more details on the evaluation of symptomatology, see and text (Patients and Methods). Variations (medium ± se) of the three parameters indicated in the five patient groups during the duration of 6 months of the trial. Variations of the remaining six clinical parameters and 4. For other details, see the legend of .Variations (medium ± se) of the three parameters indicated in the five patient groups during the 6-month period of the trial. Variations of the remaining six clinical parameters and 4. For other details, see the legend of .Variations (medium ± se) of the three parameters indicated in the five patient groups during the 6-month period of the trial. Variations of the remaining six clinical parameters are illustrated in and 3. For other details, see the legend of .Variations (medium ± se) of the three parameters indicated in the five patient groups during the 6-month period of the trial. Variations of the remaining six clinical parameters are illustrated in and 3. For other details, see . In , important variations are presented as percentage changes on the same scale for the nine clinical parameters. In these figures, the changes produced by carnitine during the third and fourth month with respect to the first and second month (i.e., the first placebo period) in patients with A2 and A4 groups are contrasted with the equivalent changes in patients in group 0 (who continued to have placebo, instead of changing to carnitine). The differences between the two carnitine groups and the placebo group were statistically significant (P 0.05), except for astenia (P Mean ± percentage change of se and 95% CI of the nine clinical parameters evaluated in patients in group 0, A2, and A4. The percentage change means the second score of the bimester or strokes per minute or kg of body weight on the first parameter of the corresponding bimester times 100. During the second quarter, patients in group 0 continued to have placebo plus T4, and their symptoms or signs continued to worsen. On the other hand, patients A2 and A4 replaced placebo with carnitine, and their symptoms or signs were melioraged. Only changes in body weight did not differ statistically (P ≤ 0.05) in group 0 vs. groups A2 or A4. Average percentage change ± and 95% CI of the nine clinical parameters evaluated in patients of group 0, A2, and A4. The percentage change means the second score of the bimester or strokes per minute or kg of body weight on the first parameter of the corresponding bimester times 100. During the second quarter, patients in group 0 continued to have placebo plus T4, and their symptoms or signs continued to worsen. On the other hand, patients A2 and A4 replaced placebo with carnitine, and their symptoms or signs were melioraged. Only changes in body weight did not differ statistically (P ≤ 0.05) in group 0 vs. groups A2 or A4. In a group, clinical amelioration began 1 or 2 wk after the carnitine administration was started. Biochemical parameters. With the exception of TSH (which was illustrated in ), phosphokinase creatine and cholesterol, the other biochemical parameters are regulated by thyroid hormones. The peripheral parameters in groups 0, A2, and A4 are illustrated in the three top panels of -8. Taking into account all data from 0 to 6 months for any given parameter, the A2 and A4 groups were statistically different from group 0 (PVariations (medium ± se) of the three biochemical parameters indicated from thyroid hormone action in the five patient groups during the 6-month period of the trial. Variations of the remaining six biochemical parameters and 8. For each biochemical parameters, all data points from 0 to 6 months in group 0 are compared to all data points in A2 and A4 or B2 and B4 groups by ANOVA, and the resulting P value is shown. In each group, the corresponding change is summarized in and 12. Variations (mean ± se) of the three biochemical parameters indicated from thyroid hormone action in the five patient groups during the duration of 6 months of the trial. Variations of the remaining six biochemical parameters and 8. For each biochemical parameters, all data points from 0 to 6 months in group 0 are compared to all data points in A2 and A4 or B2 and B4 groups by ANOVA, and the resulting P value is shown. In each group, the corresponding change is summarized in and 12. Variations (mean ± se) of the three biochemical parameters indicated from thyroid hormone action in the five patient groups during the duration of 6 months of the trial. Variations of the remaining six biochemical parameters and 8. For other details, see . To convert the cholesterol of mg/dl to mmol/l, multiply by 0.02586. Variations (mean ± se) of the three biochemical parameters indicated from thyroid hormone action in the five patient groups during the duration of 6 months of the trial. Variations of the remaining six biochemical parameters and 8. For other details, see . To convert the cholesterol of mg/dl to mmol/l, multiply by 0.02586. Variations (mean ± se) of the three biochemical parameters indicated from thyroid hormone action in the five patient groups during the duration of 6 months of the trial. Variations of the remaining six biochemical parameters and 7. For other details, see . To convert the OH-prolina urinary of mg/d toμ mol/l, multiply by 7.628. Variations (mean ± se) of the three biochemical parameters indicated from thyroid hormone action in the five patient groups during the duration of 6 months of the trial. Variations of the remaining six biochemical parameters and 7. For other details, see . To convert the OH-prolina urinary of mg/d toμ mol/l, multiply by 7.628. All these changes, standardized on a percentage basis, can be seen in , and were statistically significant (P 0.05) in groups 0 [1.0% (CI −1.7 to 3.7)], A2[ 4.0% (CI 0.4-7.5) and A4 [3.7% (CI 0.43-6.9)], as well as variations in OH-P, a bone resorption marker [3.29). On the other hand, the positively regulated osteocalcin, which is a marker of bone formation, increased to a greater extent (P 0.05). Average percentage changes (calculated as in ) and 95% of the nine biochemical parameters evaluated in groups 0, A2, and A4. Only changes in cholesterol and the OH-proline urinary line were not significant (P ≤ 0.05) in group 0 vs. groups A2 and A4. Average percentage changes (calculated as in ) and 95% of the nine biochemical parameters evaluated in groups 0, A2, and A4. Only changes in cholesterol and the OH-proline urinary line were not significant (P ≤ 0.05) in group 0 vs. groups A2 and A4. In short, carnitine has 1) an antagonistic action on the increase of thyroid hormone caused by the thyroid hormone of the serum AST, ALT, GGT, SHBG and ferritin; 2) a neutral effect on the decrease of the TSH serum caused by the thyroid hormone and total cholesterol, and the increase of the urinary excretion of OH-P; and 3) an osteoid enhancer effect Bone density. Due to the small number of subjects, the increase in post-therapeutic MMD of left lumbar column or proximal femur in groups A was not statistically significant (P = 0.05) of the corresponding post-therapy changes observed in group 0 vs. groups A (). Changes in the DMO (left vertebrae and femur) at the end of the clinical trial regarding the baseline in the five study groups. The data are presented as 95% CI, with an average ± is given in parenthesis. The differences between group 0 vs. A2 and A4 or group 0 vs. B2 and B4, analyzed by ANOVA, were not statistically significant. In a comparison to the pair, only the difference of group 0 vs. B4 in the vertebral DMO was statistically significant ( Changes in the DMO (lumbar vertebrae and left femur) at the end of the clinical trial regarding the baseline in the five study groups. The data are presented as 95% CI, with an average ± is given in parenthesis. The differences between group 0 vs. A2 and A4 or group 0 vs. B2 and B4, analyzed by ANOVA, were not statistically significant. In a comparison to pair, only group 0 vs. group B4 difference in vertebral DMO was statistically significant (Preemptive effect of carnitineSyptomatology. Modifications of the clinical parameters throughout the clinical trial in the B2 and B4 groups are illustrated in the two lower panels of Figs. 2-4, and can be contrasted with the corresponding modifications in group 0 (Figure 2-4). Except for the body weight, the 6-month profile of each parameter (i.e. general increase) in the placebo group was statistically different (P In the percentage changes based on the severity of the symptoms/signs during the 4-month adjunctive carnitine treatment in the B2 and B4 groups are contrasted with the corresponding changes in the patients of group 0, in which the adjunctive treatment was placebo. Differences between B2 and B4 vs. group 0 were significant (percentual change of PM ± and 95% CI of the nine clinical parameters evaluated in patients of group 0, B2, and B4. The percentage change means the "shared carnitine value" (i.e., score or rhythms per minutes or kg of body weight of the first 4 months) divided by the corresponding reference value (day 0), the ratio is multiplied by 100. Only changes in body weight were not significant (P 0.05) in group 0 vs. groups B2 or B4. Average percentage change ± and 95% CI of the nine clinical parameters evaluated in patients of group 0, B2, and B4. The percentage change means the "shared carnitine value" (i.e., score or rhythms per minutes or kg of body weight of the first 4 months) divided by the corresponding reference value (day 0), the ratio is multiplied by 100. Only changes in body weight were not significant (P 0.05) in group 0 vs. groups B2 or B4. Biochemical parameters. Data for the entire duration of the trial are illustrated in the upper panel (group 0) and the two lower panels (groups B2 and B4) of Figs. 6-8, while the relevant changes per cent are summarized in . Except for TSH (see ) and OH-P urinary, absolute values of the other parameters during the 6 months of the trial (Figs. 6-8) and percentage changes during the first 4 months regarding the base () 95% were in group 0 statistically different from groups B2 and B4 (P Mean ± significant changes per cent were OH4 patients) B2 and B4. Average percentage changes (calculated as in ) and 95% of the nine biochemical parameters evaluated in groups 0, B2, and B4 patients. Only changes in the urinary OH line were not significant in groups 0 vs. B2 and B4. Bone densitometry. Due to the small number of subjects, the increase in posttherapeutic MMD of left lumbar column or proximal femur in groups B was not statistically significant (P = 0.05) of the corresponding posttherapy changes observed in group 0 vs. groups B (). However, when analyzed separately the carnitine group vs. the placebo group, only the comparison between group B4 and group 0 regarding vertebral DMO was significant (P I turned events Only during the placebo administration were the following events reported: headache (two patients in group 0, one patient in group A4 and one patient in group B2, pruritus (one group 0 patient). These disturbances were described as moderate and transitory. During carnitine administration, of the 40 people in groups A and B, only one A2 patient and one B4 patient complained of riots. These consisted of nausea and gastralgia, which had appeared during the first week, disappeared in the next and were minimal, so the administration of carnitine was not suspended. Transient and moderate gastralgia was also reported by a patient group 0. Except for the changes described above for the parameters sensitive to thyroid hormone, in none of the 40 subjects there were significant alterations of the sedimentation rate of erythrocytes, blood counts (RBC, WBC, platelets), serum electrolytes (Na+, K+, Ca2emia+, P− ), serum proteins (including protein electrophoresis), bilirubinemia, creaemia, DiscussionBefore only 11 naturally hyperthyroid patients treated with 1 or 2 g/d of carnitine for up to 6 weeks had been evaluated (, , ). The clinical improvement began from the second week and consisted of a decrease in heart rate, body temperature, astenia, nervousness, insomnia, hyperreflexia and tremors. In a 4 kwk study on iatrogen hyperthyroidism (), six male volunteers who received 100 μg/d l-T3 and 1 g/d carnitine were compared to six male volunteers who received only l-T3. Unfortunately, the results are summarized in a table where a few parameters (such as media values without sd or se) are presented in different ways (), making it impossible to interpret and compare with our data. The body weight is reported as a mid-change, and was - 10.5 pounds (4.7 kg) or −10.4 pounds (−4.7 kg) in the T3 or T3 plus carnitine group, respectively. Pulse rate is reported as mean end values (98 or 90 p.m., respectively), and serum cholesterol as average change (3% or −22%) (). All these studies (–) did not specify how the symptoms were evaluated, and all were unpublished and lacked statistical analysis. With our protocol, we could evaluate whether carnitine is able to reverse and prevent (or minimize) iatrogenic hyperthyroidism. In groups A, the symptoms/signs that had worsened while in l-tiroxin more placebo returned to the base once the placebo had been replaced with carnitine. In groups B, symptoms/signs remained stable or even meliorate while carnitine was associated with l-tiroxin, which means that the carnitine effect prevailed over the T4 effect. The preponderance of the carnitine effect was confirmed by the negative sign of the second change in the first semester in groups A or the first two bimesters on the change of base in groups B that was observed for several biochemical parameters: AST, GGT, SHBG and ferritine. Therefore, our observations confirm the observations of Hellthaler et al. () in rats, in which the administration of l-tiroxine + carnitine caused a decrease of 10–50% of AST and ALT, while the administration of l-tiroxin caused an increase of 30–150%. Three biochemical parameters were saved by carnitine antagonism in thyroid hormone action: Sterile TSH, Sterile Ostecalcin and urinary OH-P. A fourth parameter, the circulating total cholesterol is relatively refractory to the carnitine, since only group B showed a statistical difference (P Due to the different effect of carnitine on osteoclasts and osteoblasts, we must expect a beneficial effect on the bone, although of low magnitude considering the relatively short period of carnitine administration and the consequent interference with only a cycle of bone formation. The beneficial effect of carnitine can be better appreciated in groups B, because they received carnitine for a longer period compared to groups A. Although in groups B the post-treatment MMD was measured in the sixth month, namely, 2 months after the withdrawal of carnitine, there was an average of 1.8% (group B2) and 2.3% (group B4) increase in the lumbar column MMD, and 1.0% (B2) and 1.3% (B4) increase in femur DMO. These beneficial changes contrasted with the changes of −0.6% and +0.2% observed in the placebo group. Due to the short duration of treatment and the small number of patients as a result of a statistically significant discharge (P When iatrogenic hyperthyroidism appears, several patients spontaneously reduce the dose of l-tiroxin or take l-tiroxin irregularly or may even stop therapy. In each case, the final result is the absence of consistent removal of TSH, and this is blurred in thyroid carcinoma patients. The traditional approach to avoid hyperthyroidism is to individually adapt the dose of l-tiroxine, but this requires frequent clinical and hormonal evaluations. (Even in doing so, some patients continue to complain of side effects, because the individual range of "eutiroids" is narrower than the population range.) These frequent controls can be removed using a fixed daily dose of TSH-suppressive l-tiroxine plus l-carnitine at 2 g a day or, as our preliminary data indicates, even 1 g a day. Alternatively, carnitine can only be added after symptoms of hyperthyroids appear. After our clinical trial ended and the results were known, patients were informed of the cost of carnitine and inquired. Patients in group A and B wondered if they were willing to continue adjunctive therapy with 2 g of carnitine a day; group 0 patients were asked if they were willing to "take twice a day and orally 2 g of a medication, as natural as thyroxine, which would have protected them from the side effects of the hormone." The proportion of the favorable response (34 out of 40 or 85% and 8 out of 10 or 80%) was similar. When the 42 favorable helpers were asked if they preferred to take carnitine for a strictly limited period of time or during the required time, 33 (79%) preferred the second modality. On a daily basis, the cost of 1 g of carnitine is $0.65, which is well compared to benzodiazepines (e.g. $0.42 to $0.6 for 3 mg brozepam or 2 mg of lorazepam) or β-blockers (e.g. $0.1 for 60 mg propanolol), taking into account that these drugs act on select symptoms and have significant side effects. Other drugs that have been used to counter hyperthyroidism are bile sequelae (–), whose cons are the high daily cost ($5 for 8 g of colestypel or 20 g), the high frequency of gastrointestinal disturbances, the impaired absorption of liposoluble vitamins and several drugs, and the main effect is limited to the first week of treatment (). Theoretically, other drugs that interfere with thyroid hormone or transport cross cells or interaction with nuclear receptors (e.g. diphenylhydantoin, nonsteroidal anti-inflammatory drugs, amiodarone, etc.) () could also be used. However, none of the previous drugs is occurring naturally, and each has important side effects. In acute contrast, carnitine does not have toxicity, teratogenicity, known contraindications, interaction with drugs or important side effects. In addition, hyperthyroidism impoverishes carnitine tissue deposits (, ), thus creating a true situation of secondary carnitine deficiency (). In our group 0 patients, 6 months of l-tiroxin therapy increased the urinary excretion of carnitine for almost 3 times (), reflecting its efflux induced by thyroid hormone of the cells. If we also consider that carnitine inhibits entry T3 and T4 in the core of a series of peripheral cells (), then there is a double rationality for the use of carnitine at least as auxiliary hyperthyroid therapy: replenish tissue deposits and counter thyroid hormones in the periphery. Carnitine may be of particular use when it is important to use the lowest possible dose of antithyroid drugs, such as pregnancy, breastfeeding and liver and/or hematological disorders (, ), as these drugs cross the placenta, are secreted in milk, and have liver and hematopoietic toxicity (, , ). Because carnitine crosses the placenta and is devoid of fetal toxicity Also the forms of thyroid hormones leakage, or antithyroid-resistant thytoxicosis (e.g. amiodarone-related thyroid-resistant thyrotoxicosis) or thyroid storm are susceptible to carnitine treatment. Thyroid storm is a serious emergency that has a 20–50% mortality and is triggered by precipitated events (). Thus, carnitine would be useful both for the prevention and therapy of thyroid storm. Recognition We are in debt to Dr. Nadia C. Aricò for statistical analysis of data and Sigma-Tau (Pomezia, Italy), especially Dr. Carlo Trevisani, for carnitine supplies. We also thank the Dres. A. Toscano and M. D'Aghennouz (Clinica Neurologica 2, Messina, Italy) for the trial of urinary carnitine. Doctors L. Bartolone and S. Squadrito participated in the early stages of this work. Abbreviations: aminotransferase alanine; aminotransferase partyry; bone mineral density; confidence interval; variation coefficient(s); free T3; free T4; γ -glutamiltransferase; glyobine sexual hormone. Cooper DS1996 Treatment of thyrotoxicosis. In: Braverman LE, Utiger RD, eds. Werner and Ingbar are the thyroid: a fundamental and clinical text, ed 7. Philadelphia: Lippincott-Raven; 713–734 Email alert s Related Articles inCiting articles via ConnectResources ExploreOxford University Press is a department at Oxford University. In addition, the University's goal of excellence in research, scholarship and education by publishing around the world or This PDF is available for Subscribers OnlyFor full access to this pdf, enter an existing account or purchase an annual subscription.

L-carnitine: Its Benefits, Sources And Side Effects - Boldsky.com

Podcast Bites - The effects of L-Carnitine on the thyroid - YouTube

Carnitine | HealthyPlace

Video: L-Carnitine In Hyperthyroidism and Graves' Disease

Is There a Way to Calm Hyperthyroidism? | The People's Pharmacy

Graves' Disease: Symptoms, Treatment of Overactive Thyroid - The Amino Company

Lower Blood Pressure, Prevent Heart Disease | Cardio Juvenate Arginine Supplement | Natural Treatment for Hyperthyroidism with L-Carnitine

L-Carnitine Benefits, Side Effects & Dosage - SelfHacked
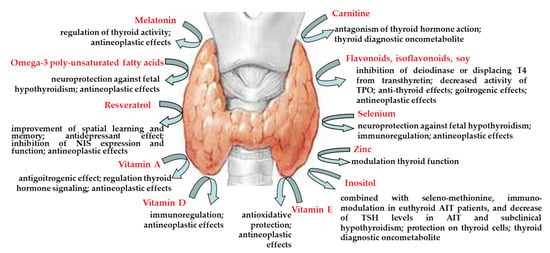
Nutrients | Free Full-Text | Nutraceuticals in Thyroidology: A Review of in Vitro, and in Vivo Animal Studies | HTML
Carnitine supplement benefit and side effects, dosage of 250 mg, 500 mg
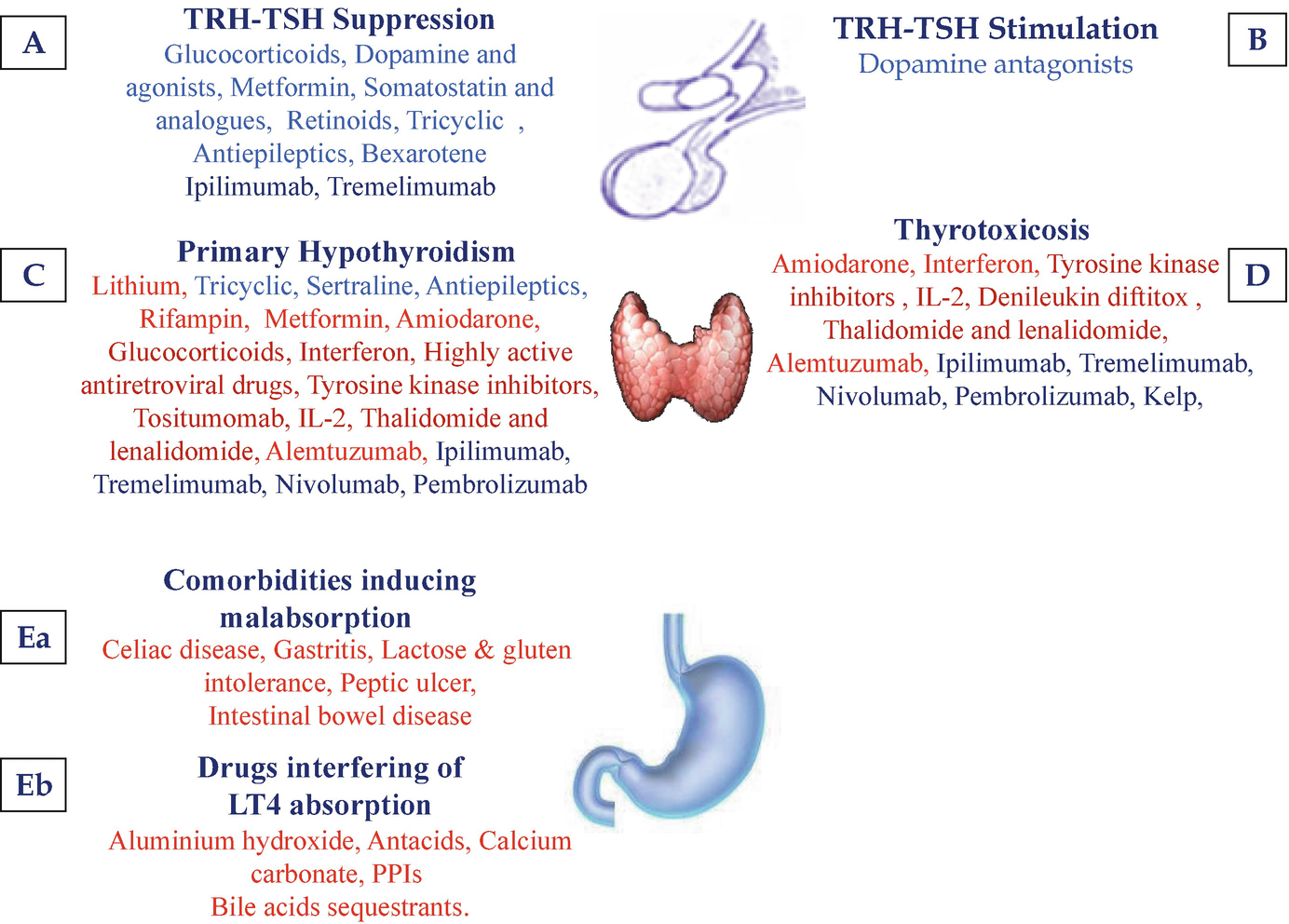
Drugs and Other Substances Interfering with Thyroid Function | SpringerLink

Carnitine and Thyroid Disease - page 1 - Life Extension
Levothyroxine (Synthroid) Supplement Interactions -- Supplements To Avoid While Taking Thyroid Hormones | ConsumerLab.com
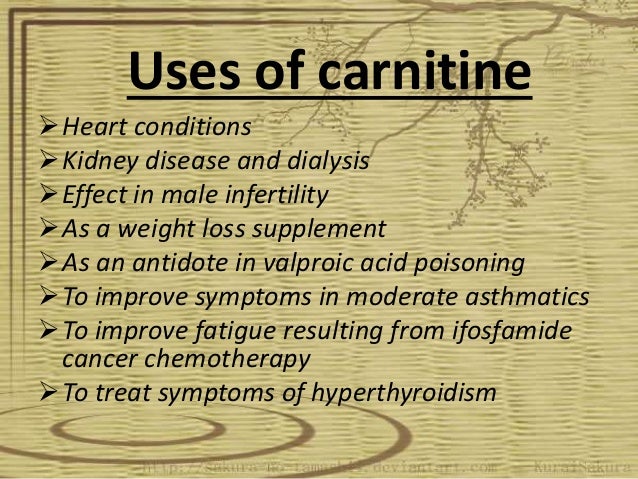
Carnitine
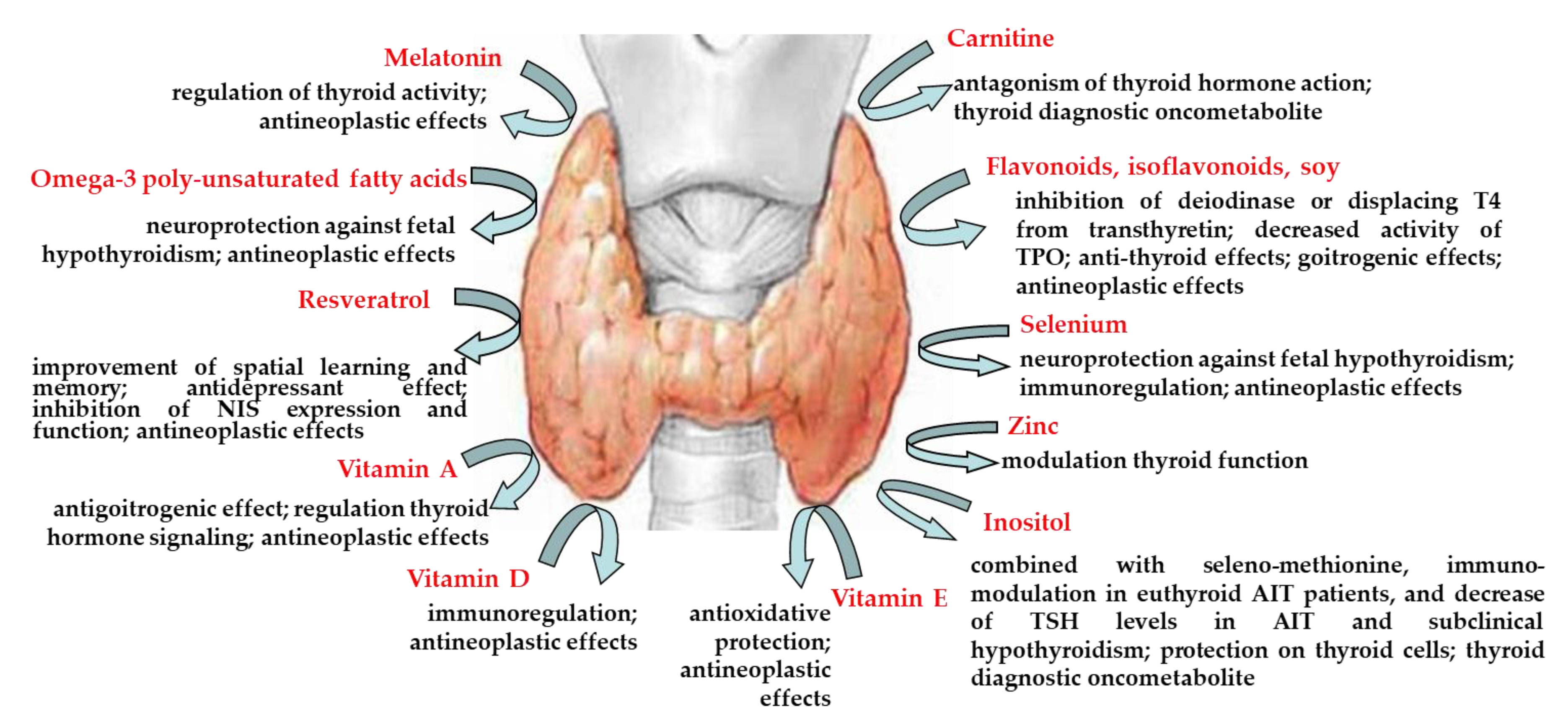
Nutrients | Free Full-Text | Nutraceuticals in Thyroidology: A Review of in Vitro, and in Vivo Animal Studies | HTML

Pin on | stayingFIT |

Acetyl-L-Carnitine 500mg 60 capsules Vitals / Vitaminedesk
Dietary and Lifestyle Interventions to Support Functional Hypothyroidism - Inquiries Journal

PDF) Clinical Concepts on Thyroid Emergencies

Successive thyroid storms treated with L-carnitine and low doses of Methimazole - The American Journal of Medicine

Graves' Disease - Episode 11 - Dr. Michael Ruscio, BCDNM, DC
![PDF] Usefulness of L-carnitine, a naturally occurring peripheral antagonist of thyroid hormone action, in iatrogenic hyperthyroidism: a randomized, double-blind, placebo-controlled clinical trial. | Semantic Scholar PDF] Usefulness of L-carnitine, a naturally occurring peripheral antagonist of thyroid hormone action, in iatrogenic hyperthyroidism: a randomized, double-blind, placebo-controlled clinical trial. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/574813b67e579e68a9bd3002d0279abfa5a0bfe6/3-Table1-1.png)
PDF] Usefulness of L-carnitine, a naturally occurring peripheral antagonist of thyroid hormone action, in iatrogenic hyperthyroidism: a randomized, double-blind, placebo-controlled clinical trial. | Semantic Scholar

Thyroid Regulation - 4 - Hormone, Subclinical Hypothyroidism, Thyroid Gland - Life Extension Health Concern | Thyroid, Hyperthyroidism, Overactive thyroid
11 Ways to Overcome Hashimoto's Fatigue - Dr. Izabella Wentz
L-CARNITINE FOR GRAVES' DISEASE AND GRAVES' OPHTHALMOPATHY An Effective Natural Treatment for Hyperthyroidism © Elaine Moor

Effects of Carnitine on Thyroid Health - Thyroid Advisor

Hyperthyroidism Natural Treatment: Foods, Supplements, and More
![PDF] Usefulness of L-carnitine, a naturally occurring peripheral antagonist of thyroid hormone action, in iatrogenic hyperthyroidism: a randomized, double-blind, placebo-controlled clinical trial. | Semantic Scholar PDF] Usefulness of L-carnitine, a naturally occurring peripheral antagonist of thyroid hormone action, in iatrogenic hyperthyroidism: a randomized, double-blind, placebo-controlled clinical trial. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/574813b67e579e68a9bd3002d0279abfa5a0bfe6/2-Figure1-1.png)
PDF] Usefulness of L-carnitine, a naturally occurring peripheral antagonist of thyroid hormone action, in iatrogenic hyperthyroidism: a randomized, double-blind, placebo-controlled clinical trial. | Semantic Scholar

The Carnitine Question: What the Research Says | Breaking Muscle

Hyperthyroidism: Diagnosis and Treatment - American Family Physician

L-Carnitine | Advanced Orthomolecular Research Inc. Canada

L-Carnitine 500 mg - 30 tablets - heart health- Congestive Heart Failure- Mitochondrial Function, Energy, Hyperthyroid, Depression, Fatigue, Athletic performance, Alzheimer's disease, peripheral arterial disease, angina pectoris, myocardial infarction ...

How Does Carnitine Support Hashimoto's? - Dr. Izabella Wentz
L-Carnitine Benefits Patients with Hypothyroidism | NHRI

Hyperthyroidism Natural Treatment: Foods, Supplements, and More

L-carnitine Supplement: Benefits, Uses, Side Effects, Dosage & Interactions

Effects of l-carnitine supplementation on weight loss and body composition: A systematic review and meta-analysis of 37 randomized controlled clinical trials with dose-response analysis - Clinical Nutrition ESPEN

Hyperthyroidism: Symptoms and Causes of Overactive Thyroid - Dr. Axe

How Thyroid Health Affects The Immune System - Renewed Vitality
Hyperthyroidism disease: Malacards - Research Articles, Drugs, Genes, Clinical Trials
Posting Komentar untuk "l carnitine for hyperthyroidism"